Fig. 22.1
Capillarization is increased in quadriceps muscle in one PH patient (a) before and (b) after exercise training (Adapted from de Man et al.[8]; Reproduced with permission of the European Respiratory Society)
Conversely, in a smaller study [19], five IPAH patients participated in a thrice-weekly strength and endurance protocol for 12 weeks and displayed improvements on multiple measures of exercise capacity. Mean SMWD improved by 58 m (p = 0.01), VE and VE/VCO2 decreased by up to 20 % (p < 0.05), and the proportion of Type IIx fibers decreased by 8 % (p = 0.05). A decrease in the proportion of these glycolytic, fatiguable muscle fibers and an increase in the proportion of Type I oxidative, fatigue-resistant fibers at the end of this study may have contributed to a higher anaerobic threshold and hence improved exercise endurance and efficiency.
In a recent study, the Mereles et al. [11] 15-week exercise protocol was implemented in order to examine the specific effects of exercise on respiratory muscle function [22]. Seven PAH patients completed the 3-week inpatient, 12-week outpatient program with excellent compliance and no serious adverse events. Mean SMWD increased at the end of the study by 81 m (p < 0.001). Measures of volitional respiratory strength including PEmax (p = 0.02) were increased, alongside a trend towards an increase in PImax. Measures of non-volitional respiratory strength including TwPmo and sniff nasal pressure also significantly increased, (p < 0.04 and p < 0.03, respectively). Increased proteolytic activity leading to atrophy of diaphragm muscle fibers as well as impaired fiber force-generating capacity of these fibers has been shown in both rats and humans [23]. Again, this may be in line with the hypothesis of increased ventilatory drive leading to muscle overuse and induction of muscle fiber loss of function [18].
Conclusions
Several barriers to efficient exercise in PH exist and are related to poor pulmonary vasculature reserve, impaired cardiac contractility, and peripheral and respiratory muscle dysfunction. As a result, prior consensus in the PH community has centered on exercise avoidance owing to concern that rigorous physical activity in the untrained patient may promote cardiopulmonary dysfunction and, possibly, sudden cardiac death. Intense, unsupervised exercise remains ill-advised in the contemporary era; however, more recent data supports beneficial effects of exercise in PH when patients are monitored, and when performed according to a disciplined and safe exercise regimen of both cardiac and strength training. Such benefits include improved cardiopulmonary fitness, quality of life, muscle strength, and even survival. An exercise prescription (Table 22.1) catered to each individual’s PH severity and comorbidities is, thus, encouraged [24].
Table 22.1
Example of exercise prescription for pulmonary rehabilitation in PH patients
Patients must be on optimized pharmacologic therapy and supplemental oxygen as needed before program enrollment |
The exercise prescription should be based on symptom assessment and 6MWT or CPET |
Exercise training should include low-intensity aerobic and strength training of the upper and lower extremities, as well as stretching, range of motion, and flexibility or respiratory muscle exercises |
Low-intensity interval training can be used |
The intensity of training can be advanced gradually to submaximal target levels, avoiding intensities leading to >70–80 % of HR reserve or peak HR higher than 120 |
Activities that lead to Valsalva-like maneuvers should be avoided to prevent sudden increases in intrathoracic pressure |
Patients’ SaO2 (arterial oxygen saturation) should be kept higher than 90 % with exercise, and SaO2 and HR monitoring should ideally be monitored continuously during exercise |
Telemetry monitoring should be considered for persons with a history of arrhythmias |
Caution must be undertaken to avoid falls for persons on anticoagulation therapy of disruption of intravenous vasodilator therapy |
Exercise should be stopped immediately if the patient develops dizziness, presyncope, chest pain, hypertension or hypotension |
Management of Pregnancy in Pulmonary Hypertension
Hemodynamic Changes in Pregnancy
During the first 2 trimesters, there is an increase in blood volume up to 50 % that, together with a decrease in both pulmonary and systemic vascular resistance, results in an increased cardiac output [25, 26]. In the pregnant patient with PAH, the elevated pulmonary vascular resistance compounded with the increase in cardiac output results in substantially increased in pulmonary arterial pressure that may progressively overwhelm the right ventricle. The peri- and postpartum stages, in particular, are associated with right ventricular weakening due to increased cardiac preload from rapid increases in cardiac venous return mediated by uterine contractions during labor, shift of uteroplacental blood flow back to the systemic circulation, and withdrawal of vena cava compression by the gravid uterus [27, 28]. By contrast, blood loss associated with either vaginal (~500 ml) or cesarean (~1000 ml) delivery resulting in acutely decreased preload occurring in the setting of right ventricular failure may promote systemic hypotension and tissue hypoxia. Ultimately, the physician must be observant of these rapid and unpredictable shifts in cardiopulmonary hemodynamics and be prepared to rapidly intervene in order to prevent a catastrophic clinical outcome.
Incidence and Outcomes
Pregnancy remains one of the major triggers for the manifestation of PAH in previously asymptomatic patients with an estimated overall incidence of 1.1 in 100,000 women [27, 29–31]. Despite the availability of current PAH-specific therapies, PAH remains a major cause of mortality in pregnancy accounting for 30–56 % of all peripartum fatalities [32, 33]. In addition, there is also a high risk for perinatal mortality of offspring born to PAH mothers given the stress on fetal growth and normal development due to reduced placental blood flow and oxygen delivery [34, 35]. Studies done prior to the availability of advanced PAH therapies have shown that elevated systemic pulmonary pressure (sPAP > 40 mmHg) and a higher NYHA functional class correlate positively with clinical deterioration and the need for an earlier delivery [36]. Premature delivery or termination of pregnancy may be necessary in some patients in the setting of clinical deterioration threatening their health and/or that of the unborn child. With the introduction of PAH-specific therapies to routine clinical practice, a reduction in PAH-related mortality during pregnancy has been observed, with one study reporting a mortality of rate of ~25 % vs. 50–70 % in the time period prior to availability of PAH-specific agents. In this study, use of advanced PAH therapies (e.g. prostanoids, ERAs) as well as calcium channel blockers in vasoreactive patients were associated with a better outcome [37]. Despite these encouraging observations, it is important to stress that PAH in pregnancy remains associated with an unacceptable rate of life-threatening complications and PAH patients should be counseled on use of available contraception methods to prevent pregnancy altogether or, if pregnant, strong consideration to early termination is often necessary.
Approach to Management of the Pregnant Patient with PAH
For the patients in whom pregnancy is pursued despite knowledge of the associated risks, it is imperative to establish an aggressive plan for frequent clinical monitoring by a multidisciplinary group that should include a PH expert, maternal-fetal medicine specialist, and cardiac anesthesiologist [38, 39]. The most frequent complications during pregnancy tend to occur during the second and early third trimester when the hemodynamic changes peak [33, 37]. In anticipation of this, visits during the first trimester should focus on assessing the need for either initiating or adjusting PAH-specific drugs as well as conventional therapies such as diuretics or anticoagulants. The latter is especially important given that pregnancy is a prothrombotic state with a fivefold risk of clotting events that could result in catastrophic outcomes in these clinically vulnerable patients [40, 41]. The choice of anticoagulant should be the result of a carefully-thought process and, when possible, in line with current consensus recommendations to minimize risks to the fetus, as some agents, such as warfarin, are associated with teratogenicity and should be avoided during early pregnancy. The most commonly used anticoagulants in pregnancy are either low or high molecular weight heparins since neither of these compound classes can cross the placental barrier [42, 43]. Despite lack of clear evidence, the general consensus is that anticoagulation should be initiated early in pregnancy, continued until the beginning to labor when it should be held to minimize blood loss during delivery period and restarted postpartum to prevent clot formation.
PAH Therapy in Pregnant Patients
Consensus guideline recommendations for determining optimal PAH-specific therapy for pregnant patients are lacking; however, currently available drugs offer a range of options of that can help the clinician optimize management of PAH during pregnancy. There are four FDA-approved available drug classes for the treatment of PAH: (1) phosphodiesterase 5 inhibitors (PDE5-I), (2) soluble guanylate cyclases (sGCs), (3) endothelin receptor antagonist (ERAs) and (4) prostanoids [44–46]. There are two currently available PDE5-I: sildenafil and tadalafil. Both agents are orally active and induce vasodilatation by increasing the availability of nitric oxide (NO) in the pulmonary circulation [47]. Use of these agents during pregnancy appears to be safe based on several published clinical reports. Riociguat, the only available sGC in the market, is a vasodilator that synergizes with NO to increase vasodilation and reduce pulmonary vascular resistance [48, 49]. Studies in animals have shown that Riociguat is teratogenic and, therefore, its use is contraindicated in pregnancy. Similarly, all available ERAs (bosentan, ambrisentan and macitentan) are contraindicated in pregnancy since use of bosentan in mice is associated with birth defects such as craniofacial malformations, patent ductus arteriosus and other vascular malformations [46, 50–52]. The most potent of the available PAH specific drugs are the prostanoids, prostacyclin analogues that induce potent pulmonary and systemic vasodilatation. The available prostanoids can be found in various formulations: treprostinil is available as an oral (Orenitram) [53], inhaled, subcutaneous and intravenous agent whereas iloprost and epoprostenol are only available in inhaled and intravenous formulations, respectively [54–56]. While not all formulations have been tested in pregnancy, there have been multiple reports of prostanoids being safely used in pregnancy to treat PAH. At present, there are no well-controlled studies to evaluate teratogenic effects of prostanoids on pregnant women, a fact that has led the FDA to assign them a pregnancy risk category B (no documented evidence of harm in humans, teratogenic effects not seen in animals). Nevertheless, manufacturers of these drugs still advise caution when using any of these compounds in pregnant patients.
Pain Control and Method of Delivery
The most challenging stages of pregnancy for the clinical team managing PAH in pregnancy are labor and delivery, in which the goals of care should be to ensure the health of both mother and child by supporting cardiopulmonary hemodynamics [36, 38]. It is imperative that fetal monitoring is instituted throughout the course of labor for early detection of signs of fetal stress requiring immediate intervention. Pain control should also be instituted early as the sympathetic surge associated with pain can result in severe cardiac stress and ischemia. The choice of anesthesia to assist with pain control must also be weighted carefully as some agents can induce both profound systemic vasodilatation and myocardial suppression resulting in a substantial fall in cardiac output and tissue hypoxia [57]. When possible, regional anesthesia should be chosen over general anesthesia as the former can provide excellent pain control without the need for intubation that the latter entails [58–60]. However, one must be aware that common forms of regional anesthesia such as epidural spinal block carry the risk of inducing hypotension due to sympathetic blockade and may adversely impact cardiopulmonary function. To avoid this complication, it is advised that the epidural anesthesia is administered slowly with while simultaneously monitoring the vitals signs of both the mother and fetus. Despite the ongoing debate concerning the optimal method of delivery, some physicians favor vaginal delivery as it presents the advantage of minimizing blood loss and preventing sudden changes in cardiac preload [61, 62]. On the other hand, cesarean delivery can be used to rapidly intervene in cases of severe fetal stress and to avoid severe hemodynamic swings induced by Valsalva maneuvers required to conduct vaginal delivery but it must be stressed that blood loss is higher with cesarean vs. vaginal delivery and this could result in hemodynamic complications ranging from hypotension to sudden cardiopulmonary collapse [39]. Finally, induction with oxytocin should be avoided as this can produce increases in pulmonary vascular resistance and tachycardia that could result in cardiopulmonary collapse and death [63, 64].
Contraception
Whether the patient is either considering or has already undergone successful pregnancy and delivery, it is imperative for the PH clinician to inform the patient of the risks associated with PAH in pregnancy and the rationale for contraception [65]. The current PAH guidelines recommend the simultaneous implementation of two different methods of contraception in female patients of reproductive age [44, 66]. Barrier methods such as condoms and spermicidal gels should not be used alone given their relatively high failure rate and should always be combined with other contraceptive methods such as the progesterone only pill, the etonogestrel-releasing subdermal implant or an intrauterine device (IUD).
Conclusion
A proposed algorithm for the management of the pregnant PH patient is shown in Fig. 22.2 [67]. It must be stressed that PAH is a strong predictor for mortality in pregnancy and should be avoided when at all possible by ensuring patient education and institution of dual contraceptive methods. In patients that choose to carry their pregnancy to term, it is imperative to assemble a multidisciplinary team that will closely monitor the patient’s clinical course. Furthermore, timely institution of PAH specific therapies to achieve optimal control of cardiopulmonary hemodynamics should help mitigate the impact of volume gain and respiratory changes associated with pregnancy. Ultimately, the most important goal of care is to ensure that both mother and fetus remain clinically stable throughout the pregnancy and delivery and that plans are in place to prevent future pregnancies.
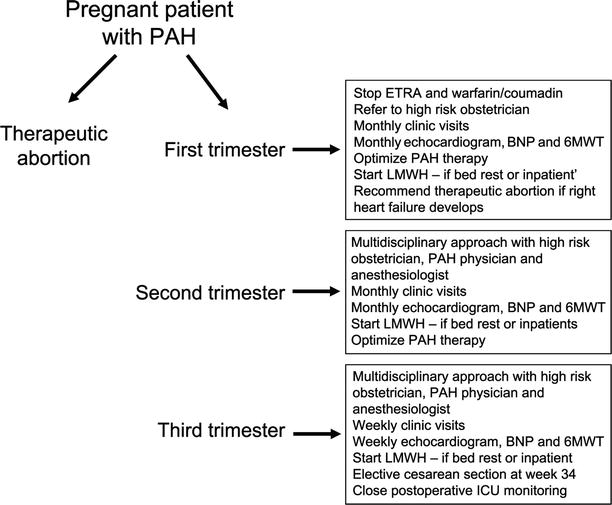

Fig. 22.2
Algorithm for the management of the pregnant patient with PH (Adapted from Zeenat Safdar et al. [67]; Reprinted by Permission of SAGE)
Perioperative Management of the Patient with Pulmonary Hypertension
Background
It is well known that the presence of moderate PH increases the risk of morbidity and mortality in both cardiac and non-cardiac surgery. One study showed that, among 2,149 patients undergoing coronary artery bypass grafting (CABG), PH was a major predictor for perioperative mortality [68]. The EuroSCORE study has also shown that PH was an independent risk factor for perioperative mortality in patients undergoing cardiac surgery [69]. Perioperative mortality and complication rates for non-cardiac surgery have been estimated to range between 7 and 42 % in one study [70], and 14 and 18 % in another [71]. While the specific risk factors that influence the incidence of life-threatening complications for PH patient in the perioperative setting are not well established, it is critical for the PH specialist to initiate a thorough management protocol that will help prepare the anesthesia and surgical team for managing acute complications related to pulmonary vascular and/or right ventricular disease.
Pre-operative Evaluation
A PH specialist should evaluate patients who require non-emergent surgery within a week of the planned surgery. This way, both patient and surgical characteristics can be thoroughly assessed and a multi-disciplinary team approach can be instituted.
Patient Characteristics
With regard to patient assessment, current functional status, comorbidities, and an evaluation of right heart function should be ascertained and updated immediately prior to surgery. New York Heart Association functional class and SWMD have been linked to PH outcomes in general [6, 72], and studies related to perioperative risk stratification have pointed to increased post-operative morbidity and mortality after surgery in NYHA class II or higher [70], and in patients with SMWD <399 m [73]. Often, the aim to improve functional class or SMWD after one office visit is an unrealistic expectation just prior to surgery, but a documented significant decline in either parameter suggests a decompensated state and is critical information that may result in the appropriate delay or cancellation of surgical intervention. Similarly, signs or symptoms of acute right heart failure such as peripheral edema, increased abdominal girth, new or worsening hypoxemia, and relative hypotension and tachycardia can be noted and treated with diuresis, supplemental oxygen and potentially augmentation of PH-specific therapy. Finally, identification of high-risk comorbidities like history of pulmonary embolism, chronic kidney disease and coronary artery disease [70, 71, 74] can assist in appropriate patient risk stratification.
A current transthoracic echocardiogram (TTE), basic laboratory tests including an N-terminal pro-basic natriuretic peptide (NT-pro BNP), an electrocardiogram (ECG) and an assessment of hemodynamics are also recommended pre-operatively [75, 76], ideally within 6 to 12 months. On TTE, poorer surgical outcomes in PH patients have been linked to right ventricular hypertrophy (RVH) and a right ventricular myocardial performance index of >0.75 [70]. On ECG, right axis deviation is associated with increased morbidity [70], while a higher right atrial pressure (RAP) [73], pulmonary artery pressure (PAP) [71, 74] and right ventricular systolic pressure/systolic blood pressure ratio of >0.66 assessed by RHC is worrisome [70]. Laboratory tests can, of course, highlight new or progressive kidney or liver injury that may represent an exacerbation of right heart failure. Moreover, new or progressive anemia or alterations in acid–base status, both potentially adversely impactful on pulmonary vascular resistance (PVR), can also be identified.
Surgical Characteristics
Details of proposed anesthetic use, surgical approach, and duration of surgery are critical variables for planning and risk stratification of surgery in PAH patients. Not surprisingly, data have shown that prolonged time under anesthesia [70, 77] and intermediate (e.g. head and neck) and high (e.g. liver transplantation) versus low (e.g. cataract) risk operations (Table 22.2, [110]) are associated with worse surgical outcomes in PH patients [71, 74, 77]. Generally, the involvement of an anesthesiologist skilled in the management of PH is advised. This often warrants the transfer of the PH patient to a tertiary care center equipped to treat PH. Although there are no strict guidelines with respect to anesthesia type (e.g. general, neuraxial) or anesthetic agent delivery method per se, careful thought must be given to the potential effects on pulmonary versus systemic vascular resistance, sympathetic tone, right ventricular (RV) loading, and cardiac inotropy and chronotropy [78]. This is because the PH patient must be considered extremely sensitive to even minor alterations in vascular tone, heart rate, gas exchange, acid–base state and volume status. For example, patients with PH can decompensate acutely and irreversibly in response to an acute increase in vagal tone (e.g. nausea during intubation) or mismatch in ventilation-perfusion (V-Q) (e.g. prone positioning) as these states can acutely decrease RV preload or induce hypoxia, respectively. Furthermore, the preoperative team need consider the appropriateness of PH-specific therapies, which is an assessment based, in part, on factors influencing effective drug delivery in the peri-operative period. A plan well in advance of surgery is ideal, one which takes into account severity of disease as well as patient comorbidities and current PH therapies.
Table 22.2
Risk stratification in PH patients by type of surgery
Low-risk operations |
Dermatologic surgeries
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|