Suppression of tumorigenicity 2 (ST2, also known as interleukin [IL]-1 receptor–like-1) is an IL-1 receptor family member with transmembrane (ST2L) and soluble isoforms (sST2). ST2L is a membrane-bound receptor, and IL-33 is the functional ligand for ST2L. sST2, a soluble truncated form of ST2L, is secreted into the circulation and functions as a “decoy” receptor for IL-33, inhibiting IL-33/ST2L signaling. Blood concentrations of sST2 are increased in inflammatory diseases and heart disease and are considered a valuable prognostic marker in both conditions. In multiple clinical trials, sST2 has emerged as a clinically useful prognostic biomarker in patients with cardiac diseases. Interestingly, sST2 even provides prognostic information in low-risk community-based populations. In this review, we will discuss analytical considerations of measuring circulating sST2 including pre-analytical issues, such as in vitro stability of sST2, biological variation of sST2, and postanalytical issues, such as reference ranges and comparisons to diseased cohorts.
Suppression of tumorigenicity 2 (ST2) is an interleukin (IL)-1 receptor family member with transmembrane (ST2L) and soluble isoforms (sST2). ST2L is a membrane-bound receptor, and IL-33 is the functional ligand for ST2L. sST2, a soluble truncated form of ST2L, is secreted into the circulation and is believed to function as a “decoy” receptor for IL-33, inhibiting the effects of IL-33/ST2L signaling. The current knowledge on ST2 biology is updated in a review by Pascual-Figal et al in this supplement of the American Journal of Cardiology .
The first enzyme-linked immunosorbent assay (ELISA) for the measurement of sST2 in human serum/plasma was constructed in 2000. Since then, sST2 serum/plasma concentrations have been extensively investigated in multiple human diseases as listed in Table 1 . In these studies, sST2 concentrations were usually associated with the respective disease, with disease severity or worse outcome. Comprehensive reviews on sST2 in different human diseases have recently been published elsewhere. The main focus of the current supplement of the American Journal of Cardiology , however, is the role of sST2 in cardiac disease. The aim of this review was to provide information on analytical considerations of measuring circulating sST2 including pre-analytical issues, such as in vitro stability of sST2, biological variation of sST2, and postanalytical issues such as reference ranges and comparisons with diseased cohorts.
| Acute complaints |
| Acute chest pain (Ref. ) |
| Acute dyspnea (Ref. ) |
| Allergy |
| Birch pollen atopics (Ref. ) |
| Arterial hypertension |
| Systemic arterial hypertension (Ref. ) |
| Pulmonary arterial hypertension (Ref. ) |
| Atherosclerosis |
| Carotid artery stenosis (Ref. ) |
| Peripheral artery disease (Ref. ) |
| Autoimmune/Rheumatoid diseases |
| Rheumatoid arthritis (Ref. ) |
| Sjögren’s syndrome (Ref. ) |
| Systemic lupus erythematous (Ref. ) |
| Wegener’s granulomatosis (Ref. ) |
| Behcet’s disease (Ref. ) |
| Dermatomyositis/Polymyositis (Ref. ) |
| Upper extremity soft tissue disorders (Ref. ) |
| Cancer |
| Acute graft-versus-host disease (Ref. ) |
| Breast cancer (Ref. ) |
| Hepatocellular carcinoma (Ref. ) |
| Malignant pleural effusion (Ref. ) |
| Cardiac disease |
| ST-elevation myocardial infarction (Ref. ) |
| Non-ST-elevation myocardial infarction (Ref. ) |
| Stable coronary artery disease (Ref. ) |
| Acute heart failure (Ref. ) |
| Chronic heart failure (Ref. ) |
| Valvular disease and cardiomyopathy (Ref. ) |
| Coronary bypass and heart surgery (Ref. ) |
| Acute cardiac allograft rejection (Ref. ) |
| Acute Kawasaki disease (Ref. ) |
| Critical illness |
| Sepsis (Ref. ) |
| Acute respiratory distress syndrome (Ref. ) |
| Trauma (Ref. ) |
| Intensive care (Ref. ) |
| Diabetes |
| Diabetes in population based studies (Ref. ) |
| Diabetes in peripheral artery disease (Ref. ) |
| Diabetes and diastolic dysfunction (Ref. ) |
| Infection |
| Bacterial (leptospirosis) (Ref. ) |
| Bacterial (tuberculosis) (Ref. ) |
| Bacterial and viral (systemic inflammatory response syndrome) (Ref. ) |
| Viral (dengue virus) (Ref. ) |
| Viral (HIV) (Ref. ) |
| Viral (influenza A) (Ref. ) |
| Inflammation |
| Human endotoxin model with LPS administration (Ref. ) |
| Inflammatory bowel disease |
| Crohn’s disease (Ref. ) |
| Ulcerative colitis (Ref. ) |
| Inflammatory skin disorders |
| Atopic dermatitis (Ref. ) |
| Bullous pemphigoid (Ref. ) |
| Liver disease |
| Acute and chronic liver failure (Ref. ) |
| Chronic hepatitis B (Ref. ) |
| Chronic hepatitis C (Ref. ) |
| Neurological disease |
| Amyotrophic lateral sclerosis (Ref. ) |
| Subarachnoidal haemorage due to ruptured aneurysm (Ref. ) |
| Pancreatitis |
| Acute Pancreatitis (Ref. ) |
| Pregnancy |
| Normal pregnancy and preeclampsia (Ref. ) |
| Miscarriage (Ref. ) |
| Reproductive medicine (Ref. ) |
| Pulmonary disease |
| Asthma (Ref. ) |
| Bronchopulmonary dysplasia (Ref. ) |
| Chronic obstructive pulmonary disease (Ref. ) |
| Idiopathic pulmonary fibrosis (Ref. ) |
| Pneumonia (Ref. ) |
| Renal disease |
| Chronic kidney disease (Ref. ) |
| Idiopathic nephrotic syndrome (Ref. ) |
| Kidney transplantation (Ref. ) |
| “Healthy” |
| Population based (Ref. ) |
| Reference value studies (Ref. ) |
The IL-33/ST2 Pathway
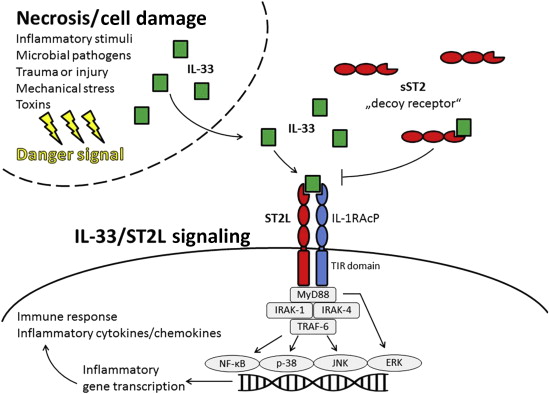
In 1989, ST2 (also known as IL-1 receptor–like-1) was first identified by 2 independent laboratories working on growth-stimulated fibroblasts. In 2005, IL-33 was recognized as the functional ligand for ST2L. IL-33/ST2L signaling has been reviewed recently and is illustrated in Figure 1 in simplified terms. IL-33 not only acts through activation of the ST2L receptor complex (shown in Figure 1 ) but also as an intracellular nuclear factor with transcriptional regulatory/repressing properties (data not shown in Figure 1 ). In principle, IL-33 functions as a danger signal or an alarm by signaling the presence of tissue damage to local immune cells after exposure to pathogens, injury-induced stress, or death by necrosis. IL-33/ST2L signaling leads then to an inflammatory gene transcription and ultimately to the production of inflammatory cytokines/chemokines and an immune response. In contrast, if sST2 binds to IL-33, it can function as a “decoy” receptor for IL-33, inhibiting IL-33/ST2L signaling. Thus, increased concentrations of sST2 in the circulation attenuate the systemic biological effects of IL-33.
The major source of circulating sST2 in healthy subjects and in patients with distinct diseases as listed in Table 1 is currently not fully established. This holds true especially for cardiac disease. For a long time, the source of circulating sST2 in cardiac disease was presumed to be myocardial following in vitro data showing load induction of ST2 messenger RNA (mRNA) in neonatal rat cardiac myocytes. More recently, however, it appears that the myocardium may not to be the major source of increased sST2 in humans with cardiac disease. However, it is clear that there is differential expression of components of the IL-33/ST2 signaling in adult human cardiac cells and cells of the vasculature. IL-33 is constitutively expressed in human cardiac fibroblasts and myocytes, whereas only minor expression of mRNA of ST2L and sST2 is found in these cells. In contrast, vascular endothelial cells might be the predominant source of mRNA expression for both ST2 isoforms and for secretion of sST2 in human cardiac disease.
Measuring Circulating sST2 and IL-33
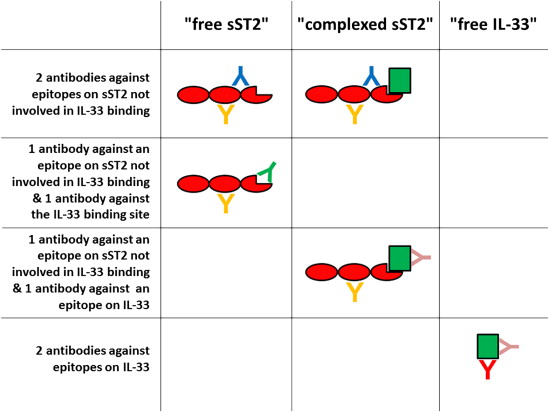
Based on the facts shown in Figure 1 , it appears that sST2 and IL-33 could be measurable in different forms in the circulation as depicted in Figure 2 . Theoretically, 3 analytes, namely “free sST2,” “complexed sST2,” and “free IL-33,” should be present in the circulation. Assuming noncompetitive assays for the detection of sST2 and IL-33 using capture and detection antibodies, different combination options are present. Depending on the antibodies used for an assay, the combination of 2 antibodies against epitopes on sST2 not involved in IL-33 binding in 1 assay would measure the sum of “free sST2” and “complexed sST2”; the combination of 1 antibody against an epitope on sST2 not involved in IL-33 binding and 1 antibody against the IL-33–binding site in an assay would quantify only “free sST2”; the combination of 1 antibody against an epitope on sST2 not involved in IL-33 binding and 1 antibody against epitope on IL-33 would detect only “complexed sST2”; and the combination of 2 antibodies against epitopes on IL-33 in 1 assay would recognize “free IL-33.” The situation is, however, even more complex because we know that different isoforms of IL-33 may be present in the circulation, and because the detection of “free IL-33” is also depending on the epitopes recognized by the respective assay antibodies (these issues are not shown in Figure 2 ).
It is unclear, but it appears likely in our opinion, that we measure the sum of “free sST2” and “complexed sST2” with the assays described in the literature. Presumably, this holds true not only for the original assay by Tominaga and coworkers but also for several published in-house assays constructed by the respective researchers and for diverse commercially available assays used in published studies on human sST2 as listed in Table 2 .
| Manufacturer | Assay/kit | Limit of detection † | Measurement range † | Intra-assay CV † | Inter-assay CV † | published articles using the assay ‡ |
|---|---|---|---|---|---|---|
| Critical Diagnostics ( www.criticaldiagnostics.com ) | Presage ST2 ST2 kit | 1.3 ng/mL | up to 200 ng/mL | <7% | <9% | Ref. |
| MBL International ( www.mblintl.com ) | Human ST2 ELISA kit | 0.032 ng/mL | up to 20 ng/mL | <6% | <6% | Ref. , |
| RayBiotech ( www.raybiotech.com ) | Human IL-1 R4/ST2 ELISA kit | 0.002 ng/mL | up to 1.2 ng/mL | <10% | <12% | Ref. |
| R&D Systems ( www.rndsystems.com ) | ST2/IL-1 R4 DuoSet ELISA or Quantikine ELISA | 0.005 ng/mL | up to 2.0 ng/mL | <6% | <8% | Ref. |
∗ There are more than 20 different ready-to-use assay kits for measurement of human sST2 commercially available. Of them, Table 2 lists only those ready-to-use assay kits that have been used in published studies on sST2 serum/plasma concentrations in human disease (effective May 2014).
† Information derived from package inserts.
‡ All published studies listed in Table 1 have been assigned to one of the four ELISAs above; with the exception of studies using home-made, non-commercially available ELISAs, and one published study using luminex technology.
Among the assays specified in Table 2 , the Presage ST2 assay (Critical Diagnostics, San Diego, California) is the only method that has been cleared by the U.S. Food and Drug Administration (FDA) and has received Conformitè Europèenne Mark. Thus, in this review, we will use the pragmatic approach to describe the analytical properties of the Presage ST2 assay first and afterward discuss its features in comparison with other methods for sST2 measurement.
Measuring Circulating sST2 and IL-33
Based on the facts shown in Figure 1 , it appears that sST2 and IL-33 could be measurable in different forms in the circulation as depicted in Figure 2 . Theoretically, 3 analytes, namely “free sST2,” “complexed sST2,” and “free IL-33,” should be present in the circulation. Assuming noncompetitive assays for the detection of sST2 and IL-33 using capture and detection antibodies, different combination options are present. Depending on the antibodies used for an assay, the combination of 2 antibodies against epitopes on sST2 not involved in IL-33 binding in 1 assay would measure the sum of “free sST2” and “complexed sST2”; the combination of 1 antibody against an epitope on sST2 not involved in IL-33 binding and 1 antibody against the IL-33–binding site in an assay would quantify only “free sST2”; the combination of 1 antibody against an epitope on sST2 not involved in IL-33 binding and 1 antibody against epitope on IL-33 would detect only “complexed sST2”; and the combination of 2 antibodies against epitopes on IL-33 in 1 assay would recognize “free IL-33.” The situation is, however, even more complex because we know that different isoforms of IL-33 may be present in the circulation, and because the detection of “free IL-33” is also depending on the epitopes recognized by the respective assay antibodies (these issues are not shown in Figure 2 ).
It is unclear, but it appears likely in our opinion, that we measure the sum of “free sST2” and “complexed sST2” with the assays described in the literature. Presumably, this holds true not only for the original assay by Tominaga and coworkers but also for several published in-house assays constructed by the respective researchers and for diverse commercially available assays used in published studies on human sST2 as listed in Table 2 .
| Manufacturer | Assay/kit | Limit of detection † | Measurement range † | Intra-assay CV † | Inter-assay CV † | published articles using the assay ‡ |
|---|---|---|---|---|---|---|
| Critical Diagnostics ( www.criticaldiagnostics.com ) | Presage ST2 ST2 kit | 1.3 ng/mL | up to 200 ng/mL | <7% | <9% | Ref. |
| MBL International ( www.mblintl.com ) | Human ST2 ELISA kit | 0.032 ng/mL | up to 20 ng/mL | <6% | <6% | Ref. , |
| RayBiotech ( www.raybiotech.com ) | Human IL-1 R4/ST2 ELISA kit | 0.002 ng/mL | up to 1.2 ng/mL | <10% | <12% | Ref. |
| R&D Systems ( www.rndsystems.com ) | ST2/IL-1 R4 DuoSet ELISA or Quantikine ELISA | 0.005 ng/mL | up to 2.0 ng/mL | <6% | <8% | Ref. |
∗ There are more than 20 different ready-to-use assay kits for measurement of human sST2 commercially available. Of them, Table 2 lists only those ready-to-use assay kits that have been used in published studies on sST2 serum/plasma concentrations in human disease (effective May 2014).
† Information derived from package inserts.
‡ All published studies listed in Table 1 have been assigned to one of the four ELISAs above; with the exception of studies using home-made, non-commercially available ELISAs, and one published study using luminex technology.
Among the assays specified in Table 2 , the Presage ST2 assay (Critical Diagnostics, San Diego, California) is the only method that has been cleared by the U.S. Food and Drug Administration (FDA) and has received Conformitè Europèenne Mark. Thus, in this review, we will use the pragmatic approach to describe the analytical properties of the Presage ST2 assay first and afterward discuss its features in comparison with other methods for sST2 measurement.
The Presage ST2 Assay
Assay format
The Presage ST2 assay is an in vitro diagnostic device that quantitatively measures sST2 in serum and plasma by ELISA. The Presage ST2 assay is FDA cleared, and as part of this, it is indicated to be used in conjunction with clinical evaluation as an aid in assessing the prognosis of patients diagnosed with chronic heart failure.
The Presage ST2 assay kit is provided in microplate configuration. The content of the kit includes a ready-to-use microtiter plate coated with mouse monoclonal antihuman sST2 antibodies (96 wells), a recombinant human sST2 standard calibrator (lyophilized), a standard diluent, an anti-sST2 biotinylated antibody reagent (mouse monoclonal antihuman sST2 antibodies) in phosphate-buffered saline, a sample diluent, a tracer concentrate and tracer diluent, a wash concentrate, a tetramethylbenzidine reagent, a stop solution, and 2 levels of controls provided in a sealed, lyophilized format (low and high control). After performing the assay procedure according to the package insert, the spectrophotometry absorbance should be read at 450 nm with a microtiter well reader. The manufacturer recommends using linear standard curve equitation. Real-time testing has revealed a shelf life of 12 months for the Presage ST2 assay kit (including the controls) when stored at 2°C to 8°C. Serum, lithium heparin plasma, and K3-EDTA plasma have been validated as possible sample types for the Presage assay.
Precision, linearity, limit of detection, and limit of quantification
The range of standards is 3.1 to 200.0 ng/ml when used with specimens diluted 1:50. The manufacturer claims a limit of blank of 0.5 ng/ml, a limit of detection of 1.3 ng/ml, and a limit of quantification of 2.4 ng/ml. In 2 published evaluation studies, a limit of detection of <2.0 ng/ml was found. The Presage ST2 assay had a within-run coefficient of variation (CV) of <2.5% and a total CV of <4.0% in one of those studies and in the other study a within-day CV of <7.6% and a total CV of <14%. Comparable results are reported by the manufacturer in the package insert. Results from linearity analyses indicate that the method is linear within the dynamic range of the assay calibration curve. These results were independently ascertained by similar experiments of the manufacturer. There are minimal effects induced by hemolysis, lipemia, icterus, or rheumatoid factor (Jaffe AS, unpublished data).
Analyte stability in vitro
The results of studies on the in vitro stability of sST2 indicate that the analyte is stable for 48 hours at room temperature, for at least 7 days at 4°C, and for at least 1.5 years at −20°C and at −80°C. Thus, the analyte as measured with the Presage ST2 assay is well suitable for routine use in laboratory settings, also facilitating unproblematic conditions for sample shipment and storage. Three freeze and thaw cycles do not effect on sST2 analyte concentrations (Jaffe AS, unpublished data).
Biological variation of sST2
The components of biological variation of sST2 in healthy subjects with a median sST2 plasma concentration of 10 ng/ml (range 5 to 34 ng/ml) were studied at 1-week intervals for 6 weeks. An intraindividual biological CV of 11%, an interindividual biological CV of 46%, and a reference change value of 30% were found. The reference change value indicates the difference required for 2 serial measurements of sST2 to be significantly different at p <0.05. This value is far lower than that of natriuretic peptides. In a similar study using the Presage ST2 assay, the authors revealed exactly the same results on the components of biological variation when blood was taken every 2 weeks for 9 weeks from subjects with a median sST2 plasma concentration of 29 ng/ml (range 12 to 75 ng/ml). The biological variation of sST2 found suggests that sST2 can be decreased by treatment over a moderate interval. This suggests that using changes of analyte concentrations might be a productive way to provide prediction of outcomes.
Fasting versus nonfasting status
To evaluate the influence of food intake on circulating sST2 concentrations, 25 diabetic and nondiabetic patients had a plasma sample drawn after an overnight fast at 7 a.m. Thereafter, all patients had a standardized breakfast (730/522 kcal). The next blood draw was done at 11 a.m. Afterward all patients had a standardized lunch (800/716 kcal). The final blood draw for this day was at 2 p.m. Mean sST2 concentrations of the 25 subjects were calculated for all 3 time points. The mean fasting sST2 concentration was 18 ng/ml at 7 a.m. , 19 U/ml at 11 a.m. , and 18 ng/ml at 2 p.m. Thus, sST2 serum/plasma concentrations appear to be independent of the fasting status.
Comparison of the Presage ST2 Assay With Other Commercially Available Assays for sST2 Measurement
Analytical method comparison
As listed in Table 2 , mainly 3 different assays (ELISAs) have been used to determine circulating sST2 concentrations in published clinical studies: the Presage ST2 assay, the MBL ST2 assay (Medical & Biological Laboratories, Woburn, Massachusetts), and the R&D ST2 assay (R&D Systems, Minneapolis, Minnesota). In contrast to the FDA-cleared Presage assay, the MBL ST2 assay and the R&D ST2 assay are research assays. The original development of the MBL ST2 assay was by the research group of Tominaga et al in Japan.
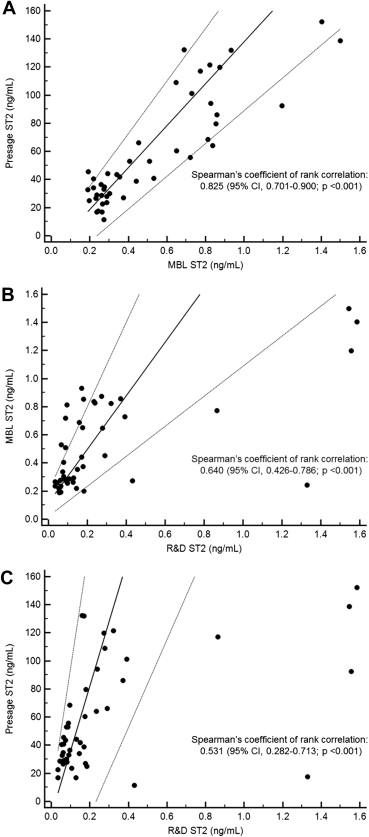
In a previously published study, sST2 plasma concentrations as measured by these 3 commercially available assays were compared. In the study participants, the median sST2 plasma concentrations were 43.5 ng/ml as measured by the Presage ST2 assay, 0.375 ng/ml by the MBL ST2 assay, and 0.144 ng/ml by the R&D ST2 assay. Regression analyses revealed that there were major differences between the 3 methods. The results of this study are summarized in Table 3 and in the scatterplots shown in Figure 3 . Concentrations of sST2 obtained with the Presage ST2 assay, the MBL ST2 assay, and the R&D ST2 assay are not equivalent. The reasons for the lack of agreement between the 3 methods are most probably different standards and/or different antibodies and, perhaps, also different reagents and buffers. Therefore, it is important to be aware that the results reported in published studies obtained with the 3 methods are not directly comparable.
| sST2 plasma concentrations as measured by the tree assays | |||||
|---|---|---|---|---|---|
| Assay | Lowest value | 25th percentile value | Median value | 75th percentile value | Highest value |
| Presage ST2 assay | 11.5 ng/mL | 28.9 ng/mL | 43.5 ng/mL | 87.8 ng/mL | 152 ng/mL |
| MBL ST2 assay | 0.189 ng/mL | 0.263 ng/mL | 0.375 ng/mL | 0.784 ng/mL | 1.500 ng/mL |
| R&D ST2 assay | 0.034 ng/mL | 0.077 ng/mL | 0.144 ng/mL | 0.274 ng/mL | 1.586 ng/mL |
| Passing and Bablok regression equations | |||
|---|---|---|---|
| Assays compared | Regression equitation | Intercept (95% confidence interval) | Slope (95% confidence interval) |
| MBL (variable x) vs. Presage (variable y) | y [ng/mL] = −11 ng/mL + 149 x [ng/mL] | −11 ng/mL (−28 to −2) | 149 (117 to 187) |
| R&D (variable x) vs. MBL (variable y) | y [ng/mL] = 0.118 ng/mL + 1.902 x [ng/mL] | 0.118 ng/mL (0.021 to 0.200) | 1.902 (1.069 to 3.000) |
| R&D (variable x) vs. Presage (variable y) | y [ng/mL] = −9 ng/mL + 459 x [ng/mL] | −9 ng/mL (−72 to 6) | 459 (312 to 891) |
∗ Plasma samples of 45 patients with a variety of diseases were measured with all three commercially available ready-to-use assay kits.
Lack of assay standardization
The currently commercially available methods for measurement of sST2 (i.e., the Presage ST2 assay, the MBL ST2 assay, and the R&D ST2 assay) are not standardized. It is unclear that any of the 3 methods has a calibrator that quantifies the analyte correctly. To resolve this issue, it would be necessary to quantify the standards of the 3 assays by the same method which should be by quantitative amino acid composition analysis.
In addition, it is not clear which epitopes are detected by the antibodies against sST2 used for the 3 methods. Therefore, it should be clarified whether the specific antibodies used in the 3 assays recognize primary, secondary, or tertiary structures of the sST2 protein. Furthermore, it depends on the purification process of the standards whether they have the appropriate molecular weight of 58,000 Da for a fully glycosylated protein. If antibodies do not recognize the primary structure epitopes of sST2, the ratio of available epitopes to the mass of protein will be dependent on retention of the structure of the epitope during the purification process of the standards. Consequently, this ratio might vary with each purification of the standard during production processes for different lots of the 3 assays.
Measuring sST2 in healthy subjects
An important issue is the capability of any given assay to accurately measure low circulating concentrations of analyte that is method dependent. It is documented that it is not feasible with the MBL ST2 assay to accurately measure sST2 concentrations in healthy subjects. The manufacturer claims a limit of detection of 0.032 ng/ml, but using the test results of 100 blood donors from the Red Cross, sST2 plasma concentrations <0.032 ng/ml were present in 83 of 100 subjects (i.e., >80%). In contrast, available data substantiate that it is possible to determine sST2 concentrations in the vast majority of healthy subjects with the Presage ST2 assay. The limit of detection of the Presage ST2 assay is <2 ng/ml as detailed previously in this review. Using test results of 528 blood donors from the Red Cross, sST2 plasma concentrations <2 ng/ml were obtained in only 8 subjects (i.e., <2% of the total sample), while in the Framingham Heart Study (a community based analysis of healthy individuals), 100% of subjects had measurable values. As a consequence, the Presage ST2 assay is considered a high-sensitivity assay for measurement of soluble ST2. No published study has evaluated the analytical sensitivity of the R&D assay. Therefore, no statement can be made whether this assay facilitates reliable measurement of sST2 concentrations in healthy subjects at low serum/plasma concentrations. Understanding distinctions between these assays is critical, as they obviously vary quite substantially with respect to their low-end sensitivity and precision.
Clinical method comparison
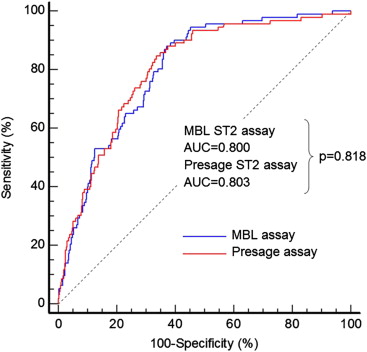
Only 1 publication provides data on the comparability of different methods for sST2 measurement in terms of clinical application. For this clinical method comparison, the ProBNP Investigation of Dyspnea in the Emergency Department (PRIDE) cohort was used. As shown in Figure 4 , of the 599 subjects in the original PRIDE cohort, 586 had blood samples available for measurement of sST2 by both the Presage ST2 assay and MBL ST2 assay. The median concentrations for the entire cohort were 27 ng/ml for the Presage assay and 0.22 ng/ml for the MBL assay. Of the 586 patients, 92 (16%) had died at 1 year and 494 (84%) survived. The receiver operating characteristic curve analyses demonstrated an area under the curve of 0.803 for the Presage assay and an area under the curve of 0.800 by the MBL assay for the prediction of death at 1 year, which were statistically similar. Hence, the clinical value of sST2 as predictor of outcome in patients with acute dyspnea is the same with the Presage ST2 assay and the MBL ST2 assay. There are no comparative data between the Presage ST2 assay and other assays for measurement of sST2.