In pregnancy, normal changes can predispose women to sleep-disordered breathing (SDB), especially those at higher risk beforehand. The goal of this section is to help clinicians identify the signs and symptoms of SDB in pregnancy and the treatment modalities available to reduce risk to both mother and fetus.
Pathophysiology and Respiratory Changes
Rising estrogen levels, as well as an increase in overall body fluids by up to 7 liters, can produce airway hyperemia and edema of the extremities and mucosa, the latter leading to rhinitis, nasal congestion, elevated Mallampati scores, and increased risk of snoring and airway obstruction. In the last trimester of pregnancy, 42% to 45% of women complain of rhinitis and nasal congestion
(22). Both estrogen and another pregnancy hormone, relaxin, cause relaxation of smooth muscles and edema leading to increased collapsibility and decreased caliber of the upper airways
(23). Pregnant women have significantly smaller upper airways compared to age-matched postpartum or nonpregnant women
(24,
25). In general, the decreased caliber of the upper airways has been linked to an increased propensity for SDB. Smaller airways lead to prolonged partial airway obstruction during sleep. The prolonged negative intrathoracic pressures during partial airway obstruction causes release of atrial naturetic peptide (ANP), reducing intravascular volume and cardiac output
(26). This, among other factors, can decrease blood flow to the placenta and oxygen delivery to the fetus, exacerbating conditions such as preeclampsia, and increasing the risk for growth restriction. Also, ANP inhibits secretion of antidiuretic hormone, leading to diuresis and increased nocturia and resulting sleep fragmentation.
Progesterone induces an increased respiratory drive and minute ventilation. These changes can lead to a natural respiratory alkalosis with a pH of 7.44 at rest
(27,
28). In addition, the growing uterus compresses the diaphragm, resulting in decreased FRC by 20% and decreased expiratory reserve volume. Th is leads to shunting, hypoxemia, and reduced oxygen stores, which could easily contribute to nocturnal respiratory disturbances such as SBD as well as compromised oxygen delivery to the fetus.
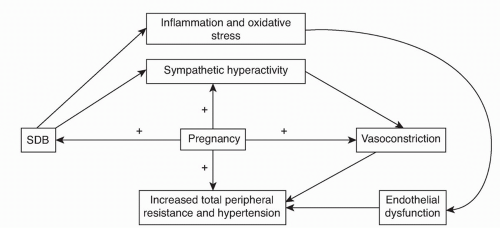
Hemodynamic oscillations associated with apneas are exacerbated during pregnancy
(29) and may contribute to maternal complications discussed later. Since sympathetic activity has been shown to be increased in the third trimester of pregnancy
(30), SDB may exacerbate this normal physiologic response, which can worsen other comorbitidies such as pregnancy-induced hypertension (PIH) and pre-eclampsia (
Fig. 19.1). The increased N1 sleep late in pregnancy may also predispose to increased frequency of central apneas
(31).
Some studies on oxygen saturations during normal late pregnancy have found them to be reduced, whereas others found them to remain stable in nonobese women. Saturations, as well as SDB, can be worse in the supine position
(19). In addition, the supine position can cause the gravid uterus to compress the vena cava leading to supine hypotensive syndrome, putting the fetus at risk of hypoxemia from uteroplacental insufficiency
(32) (
Table 19.2).
With the obvious increase in neck circumference and collapsibility of the airway, obesity can significantly worsen obstructive symptoms and increase the risk of SDB in obese mothers
(32,
33). In nonobese women, normal weight gain during pregnancy has not been proven to confer the same risk
(34).
PIH and the more severe forms of pre-eclampsia and eclampsia are associated with increased maternal and fetal morbidity and mortality and have a significant association with SDB
(28,
29,
35,
36,
37 and
38).
Many protective mechanisms also occur. For instance, pregnant women have a preference for sleeping in the lateral position, which may improve cardiac output and oxygenation as well as decrease the frequency of obstructive events
(39). An increase in minute ventilation can help to compensate for the increased oxygen demands of pregnancy but can cause symptoms of shortness of breath. As stated before, elevated levels of progesterone increase the responsiveness of upper airway dilator muscles to chemical stimuli, theoretically protecting against airway obstruction. Progesterone may also enhance respiratory drive, increasing negative pressures and causing a tendency for airways to collapse. R sleep is also decreased late in pregnancy, which may help prevent obstructive events since they are more common in this stage
(40). Finally, the fetus may be protected by enhanced oxygen delivery by the mother due to a rightward shift in the oxyhemoglobin dissociation curve.
Diagnosis
Screening for SDB should be considered in women with excessive daytime sleepiness (EDS), snoring, apneas, morning headaches, history of intrauterine growth restriction, or in the presence of hypertension or diabetes mellitus
(41).
The prevalence of SDB in pregnancy is unknown. Most of the data is from small series and case reports
(37,
42,
43,
44,
45,
46 and
47) (
Table 19.3). Review of these studies suggests that SDB may develop in women with risk factors such as obesity or may worsen the severity of pre-existing SDB, causing maternal and fetal complications discussed later
(31,
48).
The evolution of SDB over 9 months, peaking during the third trimester, is quite rapid compared to its normal progression over many years. However, the signs and symptoms of SDB in pregnant women are the same for the general population
(34). In addition, physical exam findings of obesity, increased neck circumference, and increased Mallampati scores are important factors in the diagnosis of SDB. Women with the above findings should be screened for SDB by overnight polysomnography (PSG)
(34).
Women who have PIH and pre-eclampsia risk factors should have meticulous sleep histories taken. Some have suggested that PSG should be performed in those with hypertension alone or previous pregnancies with growth restriction
(49), while others feel these are insufficient indications
(34). The consensus is that uncomplicated snoring by itself is not an indication for PSG. All of these recommendations are based on uncontrolled or nonrandomized trials and observational studies.