Fig. 13.1
Flail P2 segment from chordal rupture
Leaflet Remodeling
The leading edge of the flail or prolapsed leaflet, next to the ruptured or elongated chord, is grasped with a Resano robotic forceps and inverted into the left ventricle. This will imbricate a very small triangular shaped piece of posterior leaflet tissue to below the plane of the mitral valve annulus, ostensibly eliminating excessive redundant flail tissue. This maneuver will also present two very short near opposing curved lines of tissue along the residual posterior leaflet segment. Rolled tissue edges are then approximated with a 5-0 Gore-Tex™ suture, using a double running technique, usually for only a very short length along the body of the leaflet (Fig. 13.2). Often, a mere figure-of-eight suture will secure rapidly the inverted tissue. The imbricating suture should not be tied at this time, but tightened by slightly pulling on both ends. Usually, saline testing by pressurizing the ventricle will demonstrate elimination of the flail or prolapsing segment, restoration of a normal appearing posterior leaflet coaptation, and complete mitral valvular competence.
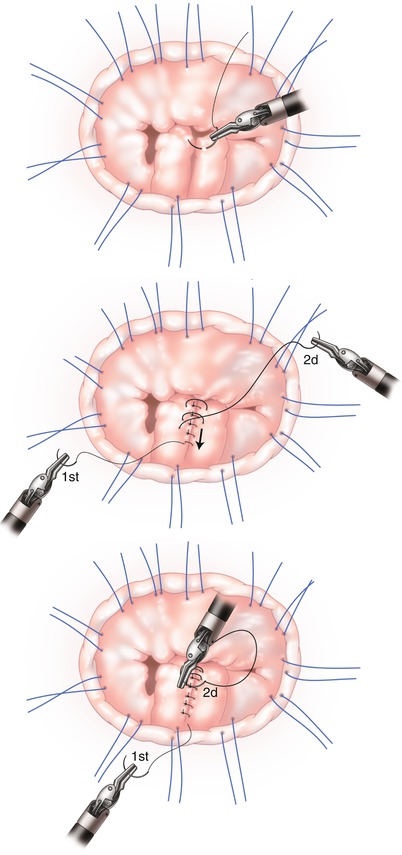

Fig. 13.2
Small portion of flail segment inverted into left ventricle. Medial edges to be approximated
Normal adjacent chords now have set the new posterior leaflet coaptation plane. Hence, neo-chordal construction and tricky length sizing is avoided. With this imbrication technique, any slight mismatch of the leading edge can be adjusted with additional passes of the suture. Moreover, if unsatisfied with leaflet remodeling, the suture can be removed and easily placed again. The fine Gore-Tex™ suture does not injure the leaflet tissue. Unlike a quadrangular resection, no irreversible actions have been committed. The suture can be tied down at any time but “suture tails” should not be cut as these ends are to be secured underneath the mitral ring prosthesis or tied onto the ring. This maneuver avoids an annoying echocardiographic appearance of artifacts and prevents leaflet injury from long mobile suture ends. An alternative, and somewhat more cumbersome option, is to invert the suture and tie on the ventricular side.
The mitral valve orifice area is then sized to select the proper ring size. After sutures are passed through the ring, sutures are tied extra-corporeally. Once the ring is implanted, mitral valve competency is again confirmed via pressure testing (Fig. 13.3). The vent, formerly in the left inferior pulmonary vein, is placed into the left ventricle, across the repaired mitral valve, and the left atriotomy is closed with a running 3-0 polypropylene suture, while de-airing the heart chambers. The cross-clamp then is removed, the heart de-aired completely under TEE guidance, and the patient removed from cardiopulmonary bypass. The remainder of the procedure is completed using standard operative methods.
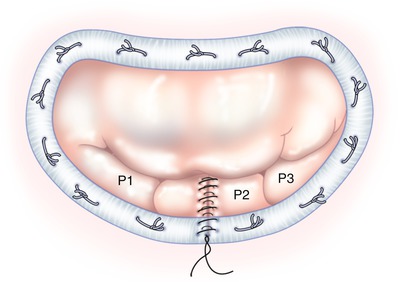

Fig. 13.3
Pressurized, competent repaired mitral valve
Tips and Pitfalls
Exposure: After positioning the left atrial retractor and prior to any leaflet reconstruction, placement of the mitral annular ring sutures is highly advantageous. When viewing the mitral valve as a “clock-face”, ventral retraction of the inter-atrial septum nearly always displaces a portion of folded posterior left atrial wall that obscures the valve from 3 to 6 o’clock. Thus, the first annular suture should be placed at the 3 o’clock position and the remainder should be placed sequentially and clockwise. This approach increases mitral valve exposure progressively as subsequent sutures placed under radial traction, flattening the obscuring atrial fold.
Inversion: Initially, we invert less tissue into the ventricle and approximate the edges with the first half of the 5-0 Gore-Tex™ suture. The valve can be pressure tested at this time. If the valve is competent, then the second half of the suture is sewn with small bites of leaflet tissue. However, if the region is still slightly prolapsed, the second half of the suture can be sewn, taking slightly larger leaflet tissue bites, and progressively inverting more prolapsed leaflet into the ventricle. We caution that imbricating too much leaflet tissue at the onset can tether the leaflet, convert the prolapse to restriction. This “fine-tuning” of imbricated tissue is a great advantage to minimally invasive surgeons and is accomplished easily with this repair technique.
Coaptation Edge: When starting the imbrication suture, the needle should be placed into matching locations in the leading edge of the leaflet to create a smooth planar surface for coaptation. Any unevenness can be corrected with the second half of the suture.
Testing: In general most surgeons test the adequacy of the mitral repair by rapidly infusing saline into the left ventricle (LV) via a bulb syringe with tubing tip placed through the mitral valve. This technique is easy and rapid, although it probably generates sub-physiologic pressures in the LV. If there is any question about the competence of the valve repair, supra-physiologic pressure testing can be easily achieved by delivering antegrade cardioplegia. When the dynamic left atrial retractor is deployed for optimal mitral valve exposure, distortion of the left fibrous trigone and aortic annulus ensues and results in aortic valve incompetence. Therefore, antegrade cardioplegia administration will generate some degree of aortic regurgitation that pressurizes and distends the left ventricle rapidly. This allows for better mitral assessment at higher LV pressures that cannot be generated by bolus instillation from the atrium.
Ring Sizing: Generally, after the leaflet pathology has been repaired and ring annuloplasty sutures are placed under radial traction, valve pressure testing should reveal a competent mitral valve repair. Then, ring sizing should be performed under full pressurization of the LV, and focused on matching the sizer with the entire mitral valve orifice area. Under sizing of the ring (as done in functional ischemic mitral regurgitation repairs) risks creating a trans-valvular pressure gradient and/or inducing leaflet distortions that may presdispose to SAM.
Deairing: Basic deairing principles are applied during sternotomy-based mitral operations. Minimally invasive and robotic mitral operations entail a few additional nuances: (1) operative field carbon dioxide insuflation throughout the operation, (2) early discontinuation of atrial, ventricular, and aortic root venting, (3) administration of antegrade and/or retrograde cardioplegia, (4) LV filling from the pump with the patient in a reversed Trendelenburg position with the left side down, and (5) valvsalva maneuvers to evacuate air from the pulmonary veins. Steps 2 through 5 should be done just as the as the atrial suture line is flushed before closure is completed. Also, a “side holed” left ventricular vent can be placed across the now competent mitral valve, allowing “holes” to reside in both the ventricle and atrium. With this method passive deairing occurs through the venting cannula during atrial closure. TEE examination during and after deairing is mandatory. Generally, when all of the above measures have been utilized routinely, almost no residual air can be observed on TEE.
Outcomes
Over 200 consecutive patients have undergone minimally invasive or robotically assisted mitral valve repair utilizing this leaflet imbrication technique. The majority had posterior leaflet P2 flail or severe prolapse. All patients were repaired successfully with leaflet imbrication and complete ring annuloplasty. There were no conversions to a sternotomy, peri-operative deaths, reoperations, or evidence of SAM. All repairs were successful on the first attempt and no patients required re-cross clamping for adjustment of a repair. On post-bypass TEE studies the vast majority of patients had zero residual mitral regurgitation after the repair and only a few patients had trace to 1+ residual regurgitation. All patients were discharged to their homes and at 1-month post-operative follow-up; all were well and free of postoperative complications [31–33].



