Desmin plays an essential role in maintaining cell cytoarchitecture, positioning and functioning of organelles, and the intercellular signaling pathway. It has been suggested that remodeling of desmin cytoskeleton might contribute to the progression of idiopathic dilated cardiomyopathy and might affect patients’ long-term prognosis. We performed endomyocardial biopsy in 200 patients with idiopathic dilated cardiomyopathy. A total of 5 to 6 specimens were collected from the left ventricular (LV) wall. Desmin was detected with immunohistochemical staining and Western blotting. Immunohistochemistry revealed 4 types of desmin expression: I, normal staining at Z-lines and intercalated disks, giving a regular cross-section pattern; IIA, increased desmin staining at Z-lines and intercalated disks; IIB, increased desmin staining with irregular pattern of cross-striation and/or with presence of aggregates; and III, decreased or lack of desmin staining. Patients with type III had a greater New York Heart Association class and N-terminal pro-brain natriuretic peptide level, larger LV end-diastolic diameter, and lower LV ejection fraction than patients with type I (p <0.001). At the end of follow-up (mean duration 59 ± 33 months), 44 patients (22%) had died and 5 (2.5%) had undergone heart transplantation. Patients with type III had an increased risk of death or heart transplantation in univariate Cox proportional hazard regression models (adjusted hazard ratio 7.18, 95% confidence interval 2.96 to 17.40, p <0.001) and multivariate models (New York Heart Association class, LV end-diastolic diameter, LV ejection fraction, N-terminal pro-brain natriuretic peptide, gender, and age; hazard ratio 5.24, 95% confidence interval 1.58 to 17.38, p = 0.007). In conclusion, in patients with idiopathic dilated cardiomyopathy, a decrease or lack of desmin expression seems to be a strong, independent predictor of an unfavorable prognosis. Our outcomes support the relevance of exploring desmin expression as a potential target to treat heart failure progression.
Idiopathic dilated cardiomyopathy (IDC) seems to be a widespread disease, representing ¼ of all dilated cardiomyopathies. Clinically, dilated cardiomyopathy is characterized by ventricular chamber enlargement, systolic dysfunction, sudden cardiac death, and heart failure (HF). HF due to dilated cardiomyopathy is a lethal disease, with a 5-year survival rate of 25% to 65%, depending on the disease stage. Currently, clinical (New York Heart Association [NYHA] class), functional (left ventricular [LV] ejection fraction [LVEF], LV end-diastolic diameter), and biochemical (N-terminal-pro-brain natriuretic peptide [NT-pro-BNP]) markers of IDC prognosis or HF are well defined; however, they do not sufficiently predict the disease course or effectiveness of selected therapies. These parameters are characterized by varying values, depending on the clinical condition and therapy. Therefore, it seems reasonable to search for new, more stable, and more specific prognostic parameters. One such parameter appears to be desmin, mainly because of its essential role in cardiomyocytes. It regulates the positioning and functioning of organelles and intercellular signalization. Because desmin plays a crucial role in cardiomyocytes, it is strongly suggested that remodeling of the desmin cytoskeleton might contribute to IDC progression. According to our knowledge, except for our previous report, no data examining such a hypothesis have been published. To test the hypothesis that desmin cytoskeleton remodeling in myocardium influences the prognosis of IDC, we performed immunohistochemical examinations of desmin expression and Western blotting protein level analysis in patients with IDC who had undergone endomyocardial biopsy.
Methods
The baseline examination was performed from January 2000 to January 2010. A total of 200 patients with IDC who had been hospitalized in our clinic were enrolled in the present study. The inclusion criteria were age ≥18 years; LVEF ≤45% as assessed by echocardiography; and clinical stability. The exclusion criteria were significant coronary artery disease found by coronary angiography (defined as the presence of any coronary artery stenotic lesion producing a > 50% reduction in lumen diameter), right bundle branch block, and effective antithrombotic therapy. The clinical, echocardiographic, and biochemical parameters were evaluated during the admission.
After enrollment, endomyocardial biopsy was performed. From each patient, 5 to 6 tissue samples (from the left ventricle) were collected using a 7F bioptome (Cordis, Johnson & Johnson, New Brunswick, New Jersey). The procedure was performed under continuous electrocardiographic monitoring.
The local ethical committee of the Central Clinical Hospital of the Ministry of Inferior Affairs and Administration (Warsaw, Poland) approved the study protocol. All study participants provided written informed consent. The study was conducted in accordance with the Helsinki Declaration.
A comprehensive, standard transthoracic echocardiographic examination was performed using a commercial diagnostic ultrasound system (iE33, Philips Medical System, Best, The Netherlands).
All measurements (e.g., LV dimensions at end-diastole and end-systole, LVEF) were performed according to the guidelines of the European Society of Echocardiography by an experienced cardiologist who was unaware of the clinical history. The LVEF, specifically, was assessed using the Simpson’s planimetric method.
The plasma concentration of NT-pro-BNP (pg/ml) was measured using an immunoassay based on electrochemiluminescence on the Cobas e411 System (Roche Diagnostic GmbH, Mannheim, Germany). The glomerular filtration rate was estimated using the Modification of Diet in Renal Disease study equation; severe renal insufficiency or kidney failure was defined as a glomerular filtration rate <30 ml/min/1.73 m 2 .
Two tissue samples from each patient were evaluated histologically with hematoxylin and eosin stain and desmin. Desmin cytoskeleton expression was investigated in formalin-fixed, deparaffinized tissue sections. The samples were incubated first with desmin monoclonal mouse anti-human antibody (1:50, DAKO, Glostrup, Denmark) and then with horseradish peroxidase-conjugated goat anti-mouse antibody (En Vision System HRP, DAKO). Negative controls were obtained by omitting incubation with the primary antibody. Immunohistochemical staining of desmin revealed 4 types: type I, normal, gentle staining of desmin at Z-lines and intercalated disks, giving a regular cross-section pattern; type IIA, increased, intensive staining of desmin at Z-lines and intercalated disks, giving regular cross-striation pattern; type IIB, increased, intensive staining of desmin with an irregular cross-striation pattern and/or with the presence of aggregates; and type III, decreased or lack of desmin staining ( Figure 1 ).
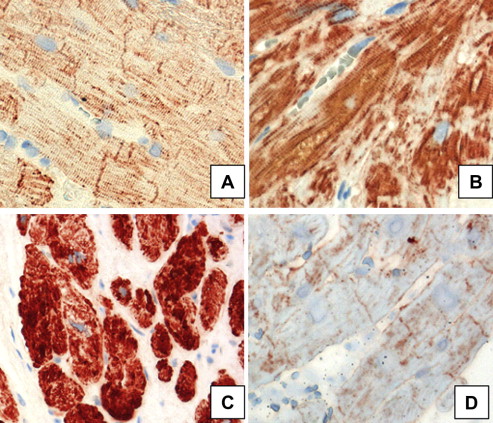
The total desmin levels in the biopsy samples were analyzed using Western blotting and were previously published.
Desmin cytoskeleton expression was evaluated in a morphometric manner. Images of whole sections were taken under the same lightning conditions and under the same optical settings using an ×4 objective with Cell p software (Olympus, Hamburg, Germany). The area equal to various desmin expression was evaluated by threshold analysis. The image threshold was determined for relative normal expression of desmin and normally distributed desmin at Z lines and intercalated discs in cardiomyocytes (type I desmin expression). Next, the images were converted to pseudocolors by fixing staining related to greater than the threshold (type II desmin expression) or lower than the threshold (type III desmin expression). Assignment of the desmin increased expression to either pattern IIA or IIB was done separately for each image using a manually fixed pseudocolor threshold. The particular type of desmin expression was measured and automatically calculated as the percentage of the total defined tissue. The dominant immunohistochemical pattern of desmin staining in the tissue sections determined the type of desmin cytoskeleton expression. Two independent pathologists evaluated the sections.
The study investigators saw patients in the HF outpatient clinic every 3 to 4 months, with a follow-up period of ≥12 months. Information regarding survival was obtained directly from patients or their relatives, the HF clinic database, or the hospital computer system. All patients completed the follow-up protocol. The primary end point was all-cause death or heart transplantation (HT) (whichever occurred first). The follow-up period for the survivors and patients with events appearing after ≥10 years was censored at 120 months.
The continuous variables with a normal distribution (age, LV end-diastolic diameter, LVEF) are presented as the mean ± corresponding SD. The intergroup differences were tested using analysis of variance and Kruskal-Wallis analysis of variance in the case of a non-normal distribution. The continuous variables with a skewed distribution (NT-pro-BNP) are presented as the median with lower and upper quartiles. The categorical variables are expressed as numbers and percentages; the intergroup differences were tested using the chi-square test. The clinical determinants of type III desmin expression in patients with IDC were established using univariate and multivariate logistic regression models, including both continuous and dichotomized determining variables. The association between the analyzed variables and survival was established using Cox proportional hazard analyses (both univariate and multivariate models). The assumptions of the proportional hazard were tested for all the covariates. To illustrate the effect of the presence of desmin expression on the 10-year event-free survival rate, Kaplan-Meier curves for cumulative survival were drawn. Differences in the event-free survival rates were tested using the Cox-Mental log-rank test. A 2-sided p value of ≤0.05 was interpreted as a statistically significant test result. For all statistical analyses, a commercial statistical package (SPSS, version 13.0, Chicago, Illinois) was used.
Results
The baseline clinical characteristics of the 200 enrolled patients and stratified by the different types of desmin expression (I, IIA, IIB, III) are listed in Table 1 . The variables that were significantly different (p ≤0.05) among the patients with type I, IIA, IIB, and III desmin expression included NYHA class, NT-pro-BNP, LV end-diastolic diameter, LVEF, and the use of β blockers, aldosterone antagonists, loop diuretics, statins, and digoxin. No differences were seen between patients with the various desmin expression types related to gender, age, body mass index, concomitant disease, and angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers. Patients with type III desmin expression had greater NYHA classes and greater NT-pro-BNP levels, larger LV end-diastolic diameters, and lower LVEFs than patients with type I (p <0.001).
| Variable | All patients (n = 200) | Type I (n = 54) | Type IIA (n = 42) | Type IIB (n = 58) | Type III (n = 46) | p Value |
|---|---|---|---|---|---|---|
| Men | 171 (85%) | 48 (89%) | 34 (81%) | 51 (88%) | 38 (83%) | NS |
| Age (yrs) | 48 ± 14 | 49 ± 16 | 47 ± 14 | 46 ± 13 | 48 ± 14 | NS |
| Body mass index (kg/m 2 ) | 27.1 ± 5.2 | 27.2 ± 4.6 | 27.4 ± 5.5 | 27.4 ± 5.8 | 26.6 ± 4.9 | NS |
| New York Heart Association class | <0.001 | |||||
| I | 53 (26%) | 28 (52%) ∗ | 9 (21%) | 11 (19%) | 5 (11%) | |
| II | 82 (41%) | 21 (39%) | 20 (48%) | 23 (40%) | 18 (39%) | |
| III | 55 (28%) | 5 (9%) | 11 (26%) | 21 (36%) | 18 (39%) | |
| IV | 10 (5%) | 0 | 2 (5%) | 3 (5%) | 5 (11%) † | |
| Left ventricular ejection fraction (%) | 30 ± 10 | 36 ± 10 ∗ | 31 ± 9 | 27 ± 8 | 27 ± 8 † | <0.001 |
| Left ventricular end-diastolic diameter (mm) | 65 ± 10 | 61 ± 9 | 64 ± 10 | 67 ± 8 | 70 ± 10 † | <0.001 |
| N-terminal-pro-brain natriuretic peptide (pg/ml) | 1,164 (489–2,760) | 400 ∗ (226–670) | 990 ‡ (556–2,043) | 2,135 § (995–3,200) | 2,758 † (1,570–4,125) | <0.001 |
| Hypertension | 90 (45%) | 28 (52%) | 16 (38%) | 26 (45%) | 20 (43%) | 0.60 |
| Hyperlipidemia | 60 (30%) | 15 (27%) | 13 (31%) | 16 (27%) | 14 (34%) | 0.85 |
| Diabetes mellitus | 30 (15%) | 6 (11%) | 6 (14%) | 9 (15%) | 9 (19%) | 0.70 |
| Renal failure Modification of Diet in Renal Disease <30 (ml/min/1.73 m 2 ) | 12 (6%) | 4 (7%) | 0 | 5 (9%) | 3 (7%) | 0.08 |
| Smoke | 57 (28%) | 14 (26%) | 13 (31%) | 14 (24%) | 13 (34%) | 0.63 |
| Medications | ||||||
| Angiotensin-converting enzyme inhibitor and/or angiotensin receptor blocker | 190 (95%) | 49 (91%) | 40 (95%) | 57 (98%) | 44 (96%) | 0.33 |
| β Blockers | 181 (91%) | 43 (80%) | 40 (95%) | 55 (95%) | 43 (93%) | 0.02 |
| Aldosterone antagonist | 135 (68%) | 25 (46%) ∗ | 31 (74%) | 49 (85%) | 30 (65%) | <0.001 |
| Loop diuretics | 146 (73%) | 37 (69%) | 26 (62%) | 42 (72%) | 41 (89%) | 0.03 |
| Statin | 86 (43%) | 25 (46%) | 25 (60%) | 20 (35%) | 16 (35%) | 0.05 |
| Digoxin | 71 (36%) | 13 (24%) | 9 (21%) ‡ | 32 (55%) | 17 (37%) | 0.001 |
| Implantable cardioverter defibrillator | 30 (15%) | 4 (8%) | 9 (21%) | 11 (19%) | 6 (13%) | 0.20 |
| Cardiac resynchronization therapy | 32 (16%) | 8 (14%) | 9 (21%) | 8 (14%) | 7 (15%) | 0.75 |
∗ p <0.05 type I vs. type IIA.
† p <0.001 type I vs. type III.
‡ p <0.05 type IIA vs. type IIB.
The mean follow-up period was 59 ± 33 months (median 51, range 1 to 120), and 44 patients (22%) died and 5 (2.5%) underwent HT. The greatest prevalence of the primary end point events (death and HT) was among the patients with type III desmin expression (24 deaths and 3 HTs [59%]). The incidence of the primary end point was sixfold greater for patients with type III expression than for patients with type I and type IIA desmin expression (6 deaths [11%] vs 4 deaths [10%], respectively) and threefold greater than for patients with type IIB (10 deaths and 2 HTs [19%]).
The mean estimated survival time free from death or HT was significantly shorter for those with type III desmin expression (66 ± 7 months, 95% confidence interval [CI] 53 to 80) than for those with other types of desmin expression (type I, 109 ± 4 months, 95% CI 101 to 117; type IIA, 105 ± 5 months, 95% CI 95 to 115; and type IIB, 93 ± 7 months; 95% CI 80 to 107).
When both all-cause death and HT were considered as events, the 10-year event-free survival rate was 62% (95% CI 52 to 73). The proportion of patients surviving 10 years free from death or HT was lower in those with type III desmin expression (28.4%, 95% CI 20% to 36.8%) than in those with types I, IIA, and IIB (83%, 95% CI 76.2% to 90.8%, 79.5%, 95% CI 70% to 89%, and 66%, 95% CI 56.8% to 75%, respectively). Statistically significant differences were seen in survival between patients with type III desmin expression and those with types I, IIA, and IIB (chi-square = 25.6, p <0.001; chi-square = 17.6, p <0.001; and chi-square = 7.1, p = 0.008, respectively; Figure 2 ).
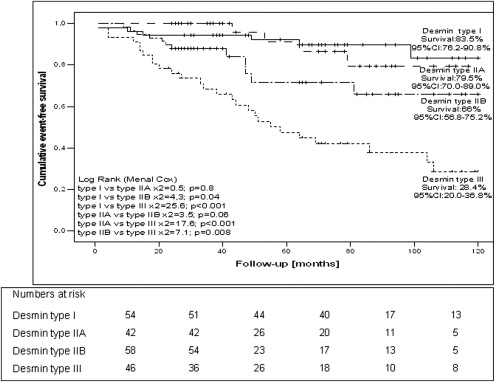
In the univariate Cox proportional hazard regression models, the following variables were shown to predict an increased rate of death and HT: older age, low NYHA class, low LVEF, high LV end-diastolic diameter, high plasma NT-pro-BNP levels, and type IIB and III desmin expression ( Table 2 ). The same variables plus male gender predicted all-cause mortality in the studied cohort ( Table 2 ). The presence of type III desmin expression in heart biopsy specimens predicted a poor outcome in patients with IDC. The univariate hazard ratio for type III desmin expression for death and HT was 7.26 (95% CI 2.99 to 17.62, p <0.001) and for death only was 6.40 (95% CI 2.61 to 15.69, p <0.001; Table 2 ).
| Prognosticator | Univariate model | Multivariate model | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | Chi-square | p Value | HR | 95% CI | Chi-square | p Value | |
| All-cause death or heart transplantation | ||||||||
| Male gender | 3.06 | 0.95–9.87 | 3.53 | 0.06 | 7.66 | 1.88–31.26 | 8.05 | 0.005 |
| Age (yrs) | 1.02 | 1.00–1.04 | 4.06 | 0.04 | 1.04 | 1.02–1.07 | 9.22 | 0.002 |
| Body mass index (kg/m 2 ) | 1.01 | 0.96–1.07 | 0.16 | 0.69 | 0.98 | 0.93–1.05 | 0.25 | 0.61 |
| New York Heart Association class | 24.75 | <0.001 | 10.28 | 0.02 | ||||
| IV vs I | 7.55 | 2.29–24.94 | 11.02 | 0.001 | 3.06 | 0.57–16.32 | 1.71 | 0.19 |
| III vs I | 6.49 | 2.62–16.09 | 16.35 | <0.001 | 2.95 | 1.00–8.66 | 3.89 | 0.05 |
| II vs I | 2.05 | 0.79–5.29 | 2.21 | 0.14 | 0.96 | 0.33–2.73 | 0.007 | 0.93 |
| Left ventricular ejection fraction (1%) | 0.94 | 0.91–0.97 | 12.07 | 0.001 | 1.00 | 0.96–1.05 | 0.009 | 0.93 |
| Left ventricular end-diastolic diameter (1 mm) | 1.07 | 1.04–1.11 | 23.46 | <0.001 | 1.05 | 1.00–1.10 | 4.19 | 0.04 |
| N-terminal-pro-brain natriuretic peptide (pg/ml) | 1.00 | 1.00–1.00 | 30.41 | <0.001 | 1.00 | 1.00–1.00 | 0.50 | 0.74 |
| Desmin type | 29.09 | <0.001 | 12.12 | 0.007 | ||||
| III vs I | 7.26 | 2.99–17.62 | 19.22 | <0.001 | 5.22 | 1.59–17.13 | 7.42 | 0.006 |
| IIB vs I | 2.99 | 1.11–8.01 | 4.72 | 0.03 | 2.36 | 0.71–7.77 | 1.98 | 0.16 |
| IIA vs I | 1.07 | 0.30–3.78 | 0.009 | 0.9 | 0.99 | 0.26–3.88 | 0.001 | 0.98 |
| All-cause death | ||||||||
| Male gender | 4.20 | 1.01–17.33 | 3.92 | 0.05 | 10.41 | 1.99–54.28 | 7.73 | 0.005 |
| Age (yrs) | 1.03 | 1.00–1.05 | 4.96 | 0.03 | 1.05 | 1.02–1.08 | 9.08 | 0.003 |
| Body mass index (kg/m 2 ) | 1.02 | 0.96–1.08 | 0.28 | 0.59 | 0.99 | 0.93–1.05 | 0.14 | 0.71 |
| New York Heart Association class | 21.74 | <0.001 | 10.29 | 0.02 | ||||
| IV vs I | 7.05 | 1.88–26.45 | 8.38 | 0.004 | 3.10 | 0.49–19.30 | 1.48 | 0.23 |
| III vs I | 7.13 | 2.67–19.10 | 15.29 | <0.001 | 3.44 | 1.07–10.99 | 4.33 | 0.04 |
| II vs I | 2.26 | 0.82–6.35 | 2.51 | 0.11 | 1.05 | 0.34–3.27 | 0.008 | 0.93 |
| Left ventricular ejection fraction (1%) | 0.95 | 0.92–0.98 | 9.04 | 0.003 | 1.01 | 0.96–1.06 | 0.07 | 0.79 |
| Left ventricular end-diastolic diameter (1 mm) | 1.07 | 1.04–1.10 | 18.43 | <0.001 | 1.05 | 0.99–1.10 | 3.29 | 0.07 |
| N-terminal-pro-brain natriuretic peptide (pg/ml) | 1.00 | 1.00 | 27.64 | <0.001 | 1.00 | 1.00–1.00 | 0.005 | 0.94 |
| Desmin type | 25.05 | <0.001 | 10.14 | 0.02 | ||||
| III vs I | 6.40 | 2.61–15.69 | 16.46 | <0.001 | 4.49 | 1.30–15.51 | 5.64 | 0.02 |
| IIB vs I | 2.44 | 0.88–6.76 | 2.93 | 0.08 | 1.81 | 0.52–6.31 | 0.85 | 0.36 |
| IIA vs I | 1.06 | 0.29–3.78 | 0.009 | 0.92 | 0.97 | 2.45–3.84 | 0.002 | 0.94 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


