Short-Term Uses of Mechanical Circulatory Support Devices
Jonathan W. Haft
Temporary mechanical circulatory support (TMCS) involves the use of artificial blood pumps to provide hemodynamic assistance for a period of hours to days to weeks. Indications may include procedural support during surgical or catheter-based interventions, postcardiotomy support for myocardial dysfunction following cardiac surgery, or resuscitation of refractory shock or cardiac arrest from a multitude of causes. At present, there are a variety of devices and techniques available to provide TMCS. These devices differ greatly in their pumping mechanisms, available applications, cannulation strategies, as well as their potential complications and adequacy of support under specific clinical circumstances. This chapter reviews the currently utilized techniques and tools for TMCS of the failing heart.
It is important to recognize the distinction between TMCS and “durable” mechanical circulatory support. Durable devices currently available in the United States include implantable left ventricular assist devices (LVAD) such as the HeartMate II, Jarvik Flowmaker, Heart Ware HVAD, HeartMate III, as well as the Syncardia Total Artificial Heart. The use of durable mechanical cardiac support has been traditionally limited to specialized centers either as a bridge to heart transplantation or for permanent use as Destination Therapy. They are most frequently indicated for patients who have chronic heart failure rather than acute cardiogenic shock. These pumps are specifically engineered for years of reliable ambulatory support and are substantially more expensive than the temporary devices discussed in this chapter. In addition, there are extensive training requirements for surgeons and the entire caregiver team. Patients in critical cardiogenic shock are not ideal candidates for durable mechanical circulatory support. This is where TMCS is ideally applied to stabilize and resuscitate, allowing time to determine if the patients will recover native cardiac function, or if the clinical circumstances are suitable for durable mechanical circulatory support.
A number of factors have to be considered when planning TMCS. Cannulation can be performed either centrally or peripherally using open surgical or percutaneous techniques. Urgency of the patient’s condition and severity of their shock may impact cannulation choice. In post-cardiotomy settings, the patient is often on cardiopulmonary bypass, allowing full circulatory assistance while transitioning to TMCS systems. However, patients in cardiac arrest on the telemetry ward or emergency department require initiation of support in the shortest interval possible, mandating a strategy that minimizes delays. Some devices have specific flow constraints, limiting their utility to scenarios that only demand partial circulatory support. Requirements for biventricular support may demand a different strategy then when patients only require support for a single ventricle. Furthermore, some patients may have cardiorespiratory failure, and thus mandate a treatment strategy which can accomplish gas exchange in addition to circulatory support. One also needs to consider the expected duration of support when choosing a device and treatment strategy that seems best suited for the clinical situation, particularly considering infectious risks and potential for patient mobility.
EXTRACORPOREAL BLOOD PUMPS
Abiomed AB 5000
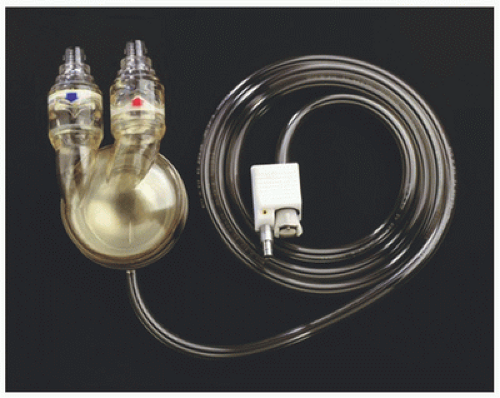
The Abiomed AB 5000 (Abiomed Inc., Danvers, MA) is an extracorporeal pneumatically driven pulsatile pump with a compliant bladder capable of producing a maximum stroke volume of approximately 100 cc (Fig. 5.1). The bladder is compressed by a surrounding air sac which actuates ejection of blood. There are polyurethane valves on the inflow and outflow conduits which ensure unidirectional flow. The air is produced by an external compressor which senses movement of air created by blood displacement. The compressor determines when the bladder has reached full expansion and initiates ejection. The AB 5000 replaces the BVS 5000 pump, which was the most frequently used temporary blood pump worldwide. The BVS 5000 pump used a similar mechanism of pneumatic actuation; however, it was associated with thrombus formation within the sinuses of the valves from stagnant flow (1). In addition, the BVS 5000 pump relied upon gravity drainage, requiring larger priming volume and made for challenging patient ambulation. The newer AB 5000 sits in a paracorporeal position, reducing prime and blood transit time. Operator prescribed vacuum assistance has been added to the external pneumatic driver, obviating the need
for gravity-based drainage. There have also been design advances within the inflow and outflow valves, improving their biocompatibility and thrombotic profile (2).
for gravity-based drainage. There have also been design advances within the inflow and outflow valves, improving their biocompatibility and thrombotic profile (2).
The Abiomed AB 5000 is provided with special cannulae for attachment to the cardiac chambers. Drainage cannulae are wire wound and malleable, allowing adaptation to the anatomic features of the chamber designated for cannulation. For left ventricular support, drainage can be accomplished in the left atrium via the right superior pulmonary vein, the interatrial groove, the left atrial dome, or the apex of the left ventricle. For right ventricular support, drainage is typically accomplished via the right atrial appendage. Reinfusion into the patient is directed into specialized cannulae bonded to woven polyester grafts for stable attachment to the ascending aorta or pulmonary artery for left and right ventricular support, respectively.
By nature of its inherent pumping mechanism, the Abiomed AB 5000 operates without specific speed setting by the clinician. Vacuum can be set to achieve the desired flow; however, the pump output automatically varies with the volume status of the patient. By functioning in a “fill to empty” mode, the pump rate will increase or decrease depending on the speed with which the bladder fills. In any individual patient, this is largely a reflection of the intravascular filling pressure of the cannulated chamber. In this way, the pump operates in an automatic mode, adjusting the output based on conditions of the patient rather than relying on a provider to determine the ideal speed. There is a mode of operation designed to be used during weaning of support, where a desired flow rate is set to allow assessment of myocardial performance under loading conditions and gradual reduction in the circulatory support provided. The AB 5000 can be used to provide left or right or biventricular support as necessary, and this versatility has made this pump, along with its predecessor the BVS 5000, well situated for application to postcardiotomy support.
Despite the improved design elements in the AB 5000, its use has gradually declined. The expansion of peripheral techniques to provide TMCS, as will be described later in this chapter, has dampened the enthusiasm for emergent sternotomy and central cannulation, as is mandated with this device. In addition, concerns have been raised about hemolysis generated by the vacuum-assisted drainage (3). Although the valve redesign has improved biocompatibility, the pulsatile nature of the pump output necessarily produces stagnant zones near the valves, and thrombogenicity remains a concern.
CentriMag
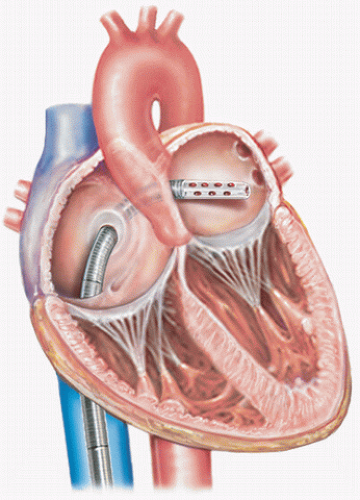
The CentriMag (Thoratec Inc, Pleasanton, CA) is a magnetically levitated centrifugal design pump with unique features that make it more durable than conventional centrifugal pumps (Fig. 5.2). This device uses magnetic levitation to allow suspension and rotation of the pump rotor without seals or bearings. While most centrifugal pumps have found medical utility as components of cardiopulmonary bypass systems (CPB), some have been used for prolonged mechanical support. However, the flow characteristics, shear forces, and heat generation create an environment that can lead to adverse events during prolonged use (4). The CentriMag was developed with these limitations in mind to create an intermediate-use rotary blood pump to provide an alternative to the pulsatile pneumatic pumps like the Abiomed AB 5000 and BVS pump. The disposable pumphead consists of a two-piece plastic outer shell and a magnetic impeller. The motor unit is reusable and generates both the magnetic field to produce rotation and radial force to suspend the rotor. There are large gaps around the rotor which are important in reducing shear forces from the turbulent secondary flows which exist in all centrifugal pumps (5). There are also no zones of flow stagnation which may predispose to thrombus formation.
The CentriMag was designed to provide short-term ventricular support in similar applications to the Abiomed AB
5000. It can be used for right ventricular assistance, left ventricular assistance, or biventricular support. The manufacturer provides a 34F drainage cannula which is wire reinforced to be kink resistant, but is malleable, allowing flexible manipulation to gain access to a variety of central cannulation sites. There is also a 24F reinfusion cannula with accessories to allow either direct insertion or use of Seldinger technique. The console was also conceived with prolonged intensive care unit support in mind. Its small footprint and simplistic push button interface with integral alarms differ favorably from the larger consoles of traditional centrifugal pumps which are intended to be operated with the continuous presence of a CPB perfusionist.
Clinical use of the CentriMag for cardiogenic shock has been favorable overall (6). The initial report from Harefield Medical Center, UK, demonstrated excellent pump performance with no hemolysis and no evidence of pump-related thrombus. In the US multicenter pilot trial, 38 patients underwent open-label nonrandomized implantation of a CentriMag for cardiac failure (7). Twelve patients received right ventricular assistance only for right ventricular failure following implantation of a durable LVAD. Fourteen patients who presented in shock following acute myocardial infarctions underwent implantation with biventricular devices (8) or CentriMag LVAD only (1). Twelve patients required postcardiotomy support, with five as biventricular assistance and seven with isolated LVADs. Overall survival was 42%. Adverse events were common, as expected in this patient population. There were no cases of pump failure or malfunction. There were two cases of hemolysis (5%), thought to be cannula related rather than attributed to the pump itself. There was one (3%) patient who had an embolic cerebrovascular accident felt to be pump related. Takayama and colleagues (9) reported a large single-institutional series of 143 patients undergoing CentriMag implantation. There were no device malfunctions or device thrombosis. There were two cases of clot within the external tubing requiring component exchange. Overall survival to hospital discharge was 57%, the highest in non-postcardiotomy shock (66%) or in failing heart transplants (73%). Development of renal failure was independently associated with death.
Based on published clinical use, the CentriMag appears to have improved biocompatibility with reduced need for pump exchange and lower incidence of thromboembolic complications and minimal hemolysis, as compared to clinical experience with the AB 5000. Its use for postcardiotomy support has increased substantially because of its ease of use and superior durability. However, there are some drawbacks to centrifugal design pumps for short-term circulatory assistance which must be considered. These devices operate at a fixed rotational speed. Although there may be some limited intrinsic responsiveness to changes in preload, providers must be aware that manual adjustments in speed may be required as patient conditions change. For example, a progressive rise in intravascular volume from transfusion or fluid administration may result in congestion if the pump speed is not increased accordingly. On the other hand, a drop in venous return from diuresis, bleeding, or tamponade can result in suction generated by the pump, leading to hemolysis, vascular trauma, or, in extreme cases, cavitation. Unlike the pulsatile pumps which operate automatically based on sensing of bladder fill, continuous flow centrifugal pumps must have the adequacy of their pump speed carefully monitored by echocardiography, intracardiac filling pressures, or astute clinical acumen of an experienced provider.
PERIPHERAL VENTRICULAR ASSISTANCE
TandemHeart
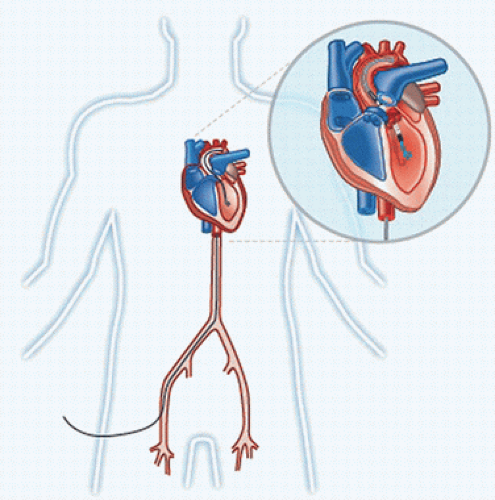
The TandemHeart percutaneous left ventricular assist device (pVAD) system (Cardiac Assist Inc, Pittsburgh, PA) consists of a unique centrifugal design pump along with a venous cannula designed for percutaneous transeptal drainage of the left atrium via the femoral vein (Fig. 5.3). The centrifugal pump is designed to produce an extremely small footprint allowing
placement immediately adjacent to the patient, reducing profile and prime. This is accomplished by integrating the pump motor and pumphead housing into a sterile single-use unit. The rotor is suspended by a bearing which is lubricated and cooled by a continuous infusion of saline, potentially reducing thrombus formation and hemolysis. The transeptal cannula is specifically designed for insertion via the femoral vein across the interatrial septum into the left atrium. The cannula is created with a curved distal tip for stable placement, and the array of side holes is located distally to prevent cyanosis from inadvertent drainage of right atrial blood. Flow is returned via a 15F or 17F arterial cannula which is wire reinforced to prevent obstruction. The entire system is inserted percutaneously using fluoroscopic and echocardiographic guidance.
placement immediately adjacent to the patient, reducing profile and prime. This is accomplished by integrating the pump motor and pumphead housing into a sterile single-use unit. The rotor is suspended by a bearing which is lubricated and cooled by a continuous infusion of saline, potentially reducing thrombus formation and hemolysis. The transeptal cannula is specifically designed for insertion via the femoral vein across the interatrial septum into the left atrium. The cannula is created with a curved distal tip for stable placement, and the array of side holes is located distally to prevent cyanosis from inadvertent drainage of right atrial blood. Flow is returned via a 15F or 17F arterial cannula which is wire reinforced to prevent obstruction. The entire system is inserted percutaneously using fluoroscopic and echocardiographic guidance.
A prospective multicenter randomized trial was conducted comparing the efficacy of the TandemHeart pVAD versus an intra-aortic balloon pump for patients in cardiogenic shock (10). Thirty-three patients were randomized at 12 different centers. Patients treated with the TandemHeart saw greater increase in cardiac output and mean arterial pressure as well as reduction in pulmonary capillary wedge pressure. There was no difference in overall survival or rates of weaning from either strategy.
Kar (11) reported a large single-institution series of patients undergoing TandemHeart pVAD for cardiogenic shock. In their series of 117 patients, most were on intra-aortic balloon pumps, required multiple vasopressors, and nearly half had required cardiopulmonary resuscitation (CPR). Average flow rates from the device were 3.3 L/min. Postimplant cardiac index increased to 2.8 L/min/m2, heart rate fell from 103 to 85, and lactate levels were reduced by half within 24 hours. Thirty-one patients were transitioned to durable LVADs and five patients underwent heart transplantation. Overall survival was 60% at 30 days. Average duration of TandemHeart support was approximately 6 days. Need for CPR was an independent predictor of death.
The TandemHeart pVAD system, when compared to surgically placed extracorporeal blood pumps has the attractive feature of peripheral insertion, reducing bleeding risks and increasing the speed with which support can be initiated. However, there are potential drawbacks. This device is currently only capable of providing left ventricular assistance; so for patients with biventricular failure, the TandemHeart may not adequately reverse cardiogenic shock. Percutaneous insertion in the femoral vessels prevents patient ambulation causing additional debility and limiting any degree of functional rehabilitation while awaiting more definitive treatment. There is also the hazard of limb ischemia from the large-size cannula obstructing distal flow in the femoral artery, although the reported incidence was low. The venous cannula can become malpositioned with only a several-centimeter window in which the drainage side holes must reside entirely within the left atrium. Nursing and patient education must be strictly enforced to prevent inadvertent withdrawal and the resulting cyanosis. There is also a concern that excessive unloading of the dysfunctional left ventricle could create an environment with absent ejection producing stasis and thrombosis within the ventricle or aortic root (Fig. 5.4). Most users of the TandemHeart recommend pump speed setting that allows some degree of left ventricular ejection and arterial pulsatility. The largest barrier to more widespread adoption of the Tandem-Heart pVAD system has been the availability of interventional cardiologists with the specialized skills required for insertion of the transeptal cannula.
 Get Clinical Tree app for offline access 
|