Drugs for erectile dysfunction (ED) may be contraindicated with nitrates commonly used to treat patients with angina pectoris, and certain antianginal therapies may worsen ED. The American Heart Association and the Princeton Consensus Conference panel of experts recommend that patients with coronary artery disease and ED who experience angina pectoris undergo full medical evaluations to assess the cardiovascular risks associated with resuming sexual activity before being prescribed therapy for ED. Current antianginal therapies include β blockers, calcium channel blockers, short- and long-acting nitrates, and ranolazine, a late sodium current inhibitor. Short- and long-acting nitrates remain a contraindication with phosphodiesterase-5 inhibitors commonly used to treat patients with ED, and the benefits of the other antianginal therapies must be weighed against their effects on cardiovascular health and erectile function. In conclusion, patients with coronary artery disease and ED who wish to initiate phosphodiesterase-5 inhibitor therapy and need to discontinue nitrate therapy need treatment options that manage their angina pectoris effectively, maintain their cardiovascular health, and provide the freedom to maintain their sexual function.
Patients with coronary artery disease (CAD) often have erectile dysfunction (ED), including severe ED, and similarly, patients with ED often have CAD and risk factors for CAD. In a study of 132 men who underwent angiography, 40% had experienced ED before being diagnosed with CAD. Indeed, the prevalence of ED has been positively correlated with risk factors for CAD, including hypertension, dyslipidemia, smoking, diabetes, and lack of physical activity. A recent meta-analysis evaluating the predictive ability of ED for cardiovascular (CV) events found that ED was associated with an increased risk for CV events and all-cause mortality. The magnitude of the risk was found to be similar to that of established predictors of CV disease risk, such as hypertension and dyslipidemia. In patients with CAD and ED, physical activity (including sexual activity) may trigger cardiac events, including angina pectoris (AP), an underlying symptom of CAD. Development of AP due to physical activity typically depends on the vigor of the activity, with patients unable to maintain 3 to 5 metabolic equivalents of exertion (METs) during an exercise test without AP or ST-segment depression being at increased risk for coital-related AP. Careful evaluation of CV risk is necessary before physical or sexual activity can be resumed, particularly in patients who need ED therapy. In this review, we examine the impact of AP on sexual activity in patients with CAD and evaluate the pharmacologic treatment options available to patients with CAD and ED that allow maintenance of sexual activity with minimal impact on CV health.
Coital Angina Pectoris
Coital AP, or AP that occurs during sexual activity, represents <5% of all reported AP attacks and is more prevalent in men than in women. Patients with CAD who experience coital AP often have a fear of recurrence that results in a lower frequency or avoidance of sexual activity and a decrease in quality of life.
In an evaluation of the association between sexual activity and the triggering of a myocardial infarction, a meta-analysis of 4 studies involving 2,960 patients found that sexual activity was associated with an increased risk for myocardial infarction (relative risk 2.70, 95% confidence interval 1.48 to 4.91, p = 0.001). Although sexual activity was found to be associated with an increased relative risk for CV events, these occurrences were more common in patients with severe CAD who engaged in lower levels of physical activity and who experienced AP with minimal physical activity.
The American Heart Association treatment guidelines recommend that patients with CAD wishing to resume sexual activity undergo thorough clinical evaluations, complete with medical history and physical examination, to determine the potential CV risk of resuming sexual activity ( Table 1 ). In addition, the guidelines specifically suggest that patients capable of exercising at 3 to 5 METs without AP, excessive dyspnea, ischemic ST-segment changes, cyanosis, hypotension, or arrhythmia after clinical evaluation can reasonably resume sexual activity. In contrast, sexual activity should be deferred in patients with unstable or refractory AP until their condition is stabilized and optimally managed. Along similar lines, given the overlap in risk factors for CAD and ED (diabetes, hypertension, hyperlipidemia, and heart failure) and the common pathophysiology mediated through endothelial dysfunction, the Princeton Consensus Conference panel of experts recommends the evaluation and management of risk factors in patients with ED, with and without CAD, and has developed an algorithm to assess CV risk ( Figure 1 ).
| Condition | Recommendation |
|---|---|
| No or mild angina | Sexual activity is reasonable (class IIa, level of evidence B) |
| Uncomplicated myocardial infarction | Sexual activity is reasonable ≥1 week after attack if patient is without cardiac symptoms during mild to moderate physical activity (class IIa, level of evidence C) |
| Complete coronary revascularization or noncoronary open heart surgery | Sexual activity is reasonable for patients who have undergone complete coronary revascularization (class IIa, level of evidence B) and may be resumed (1) several days after percutaneous coronary intervention if the vascular access site is without complications (class IIa, level of evidence C) or (2) 6 to 8 weeks after standard coronary artery bypass graft or noncoronary open heart surgery, provided the sternotomy is well healed (class IIa, level of evidence C) |
| Incomplete coronary revascularization | Exercise stress testing can be considered to assess the extent and severity of residual ischemia (class IIb, level of evidence C) |
| Unstable or refractory angina | Sexual activity should be deferred for patients with unstable or refractory angina until their conditions are stabilized and optimally managed (class III, level of evidence C) |
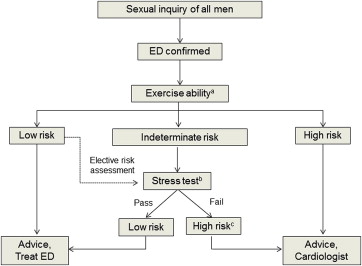
Guidelines issued by the Princeton III Consensus Conference recommend that all men with ED undergo full medical assessments of exercise ability and stress testing to establish levels of CV risk, to ensure that every patient has the CV health necessary to successfully engage in sexual activity before prescribing treatment for ED. Patients determined to be at low risk (in whom sexual activity does not represent significant cardiac risk), including those with successful revascularization, controlled hypertension, mild valvular disease, and New York Heart Association class I and II left ventricular dysfunction or heart failure who achieved 5 METs without ischemia on recent exercise testing, can initiate or resume sexual activity and begin ED treatment without further testing or evaluation. Intermediate-risk patients, including those with mild or moderate stable AP, New York Heart Association class III congestive heart failure, and histories of stroke or transient ischemic attack, should undergo further evaluation before resuming sexual activity and undergoing treatment for ED. High-risk patients, including those with unstable or refractory AP, uncontrolled hypertension, and New York Heart Association class IV congestive heart failure, should defer all sexual activity and stabilize their cardiac conditions before resuming sexual activity and initiating treatment for ED.
Phosphodiesterase-5 Inhibitors for Erectile Dysfunction
In a survey of 76 male outpatients in a cardiology practice, approximately 75% of the men responding to the Sexual Health Inventory for Men questionnaire reported having ED. The most commonly prescribed and widely used oral drug therapy for ED is phosphodiesterase-5 inhibitors, and in 2006, sildenafil and tadalafil, the first and second phosphodiesterase-5 inhibitors approved in the United States for the treatment of patients with ED, were the 32nd and 74th most popularly dispensed prescription therapies, respectively. Sildenafil, tadalafil, and vardenafil are recommended as first-line therapies for ED by the American Urological Association. Avanafil, the newest phosphodiesterase-5 inhibitor on the market in the United States, was approved for the treatment of ED in 2012. Phosphodiesterase-5 inhibitors all have the same mechanism of action and act by reducing the degradation of cyclic guanosine monophosphate by phosphodiesterase-5, promoting vasodilation and blood flow into the penis and restoring erectile function. Because of these mechanistic similarities, phosphodiesterase-5 inhibitors have comparable safety and efficacy profiles, although differences in their half-lives may be of cardiac clinical importance.
A review of data from manufacturers’ randomized, placebo-controlled trials and postmarketing safety databases showed that sildenafil (at doses of 50 and 100 mg) was generally well tolerated. There was a higher incidence of common adverse events associated with phosphodiesterase-5 inhibition, such as headache, vasodilation, and facial flushing, in patients receiving sildenafil compared with those receiving placebo. However, these rates were similar to rates previously reported in clinical studies, and further review of the postmarketing safety data showed that sildenafil did not increase the rate of myocardial infarction or other serious CV events in men with ED. In a randomized, placebo-controlled study of the effect of sildenafil on exercise tolerance in patients with CAD and ED experiencing chronic stable AP, the addition of sildenafil was noninferior to placebo with respect to time to onset of AP. This result suggested that the addition of phosphodiesterase-5 inhibitors to non-nitrate-based antianginal regimens would not impair exercise tolerance gains in patients with ED and stable AP. Data from pooled analyses of several dozen trials and thousands of patients showed that treatment with phosphodiesterase-5 inhibitors, including tadalafil and vardenafil, did not affect the incidence of CV events in patients with CAD. Avanafil is not recommended in patients with previous myocardial infarction, arrhythmia, or coronary revascularization within the past 6 months, with unstable AP, coital AP, or New York Heart Association class II or greater congestive heart failure.
Phosphodiesterase-5 Inhibitors for Erectile Dysfunction
In a survey of 76 male outpatients in a cardiology practice, approximately 75% of the men responding to the Sexual Health Inventory for Men questionnaire reported having ED. The most commonly prescribed and widely used oral drug therapy for ED is phosphodiesterase-5 inhibitors, and in 2006, sildenafil and tadalafil, the first and second phosphodiesterase-5 inhibitors approved in the United States for the treatment of patients with ED, were the 32nd and 74th most popularly dispensed prescription therapies, respectively. Sildenafil, tadalafil, and vardenafil are recommended as first-line therapies for ED by the American Urological Association. Avanafil, the newest phosphodiesterase-5 inhibitor on the market in the United States, was approved for the treatment of ED in 2012. Phosphodiesterase-5 inhibitors all have the same mechanism of action and act by reducing the degradation of cyclic guanosine monophosphate by phosphodiesterase-5, promoting vasodilation and blood flow into the penis and restoring erectile function. Because of these mechanistic similarities, phosphodiesterase-5 inhibitors have comparable safety and efficacy profiles, although differences in their half-lives may be of cardiac clinical importance.
A review of data from manufacturers’ randomized, placebo-controlled trials and postmarketing safety databases showed that sildenafil (at doses of 50 and 100 mg) was generally well tolerated. There was a higher incidence of common adverse events associated with phosphodiesterase-5 inhibition, such as headache, vasodilation, and facial flushing, in patients receiving sildenafil compared with those receiving placebo. However, these rates were similar to rates previously reported in clinical studies, and further review of the postmarketing safety data showed that sildenafil did not increase the rate of myocardial infarction or other serious CV events in men with ED. In a randomized, placebo-controlled study of the effect of sildenafil on exercise tolerance in patients with CAD and ED experiencing chronic stable AP, the addition of sildenafil was noninferior to placebo with respect to time to onset of AP. This result suggested that the addition of phosphodiesterase-5 inhibitors to non-nitrate-based antianginal regimens would not impair exercise tolerance gains in patients with ED and stable AP. Data from pooled analyses of several dozen trials and thousands of patients showed that treatment with phosphodiesterase-5 inhibitors, including tadalafil and vardenafil, did not affect the incidence of CV events in patients with CAD. Avanafil is not recommended in patients with previous myocardial infarction, arrhythmia, or coronary revascularization within the past 6 months, with unstable AP, coital AP, or New York Heart Association class II or greater congestive heart failure.
Pharmacotherapy in Patients With Chronic Angina Pectoris
Current therapies for patients with chronic AP include β blockers, calcium channel blockers, short- and long-acting nitrates, and ranolazine, a late sodium current inhibitor. Organic nitrates are often used for the treatment of chronic AP, and patients with CAD are often on nitrates, either short- or long-acting or both. Sublingual nitroglycerin (tablet or spray) is the most commonly prescribed therapy for AP attacks and has a rapid onset of action (<1 to 3 minutes). Nitroglycerin can also be used prophylactically if the onset of AP attack can be reliably predicted. Longer-acting nitrates (patches, ointment, and oral nitrates such as isosorbide dinitrate and mononitrate) are available in convenient doses given every 8 to 12 hours and can be used as long-term treatment for AP.
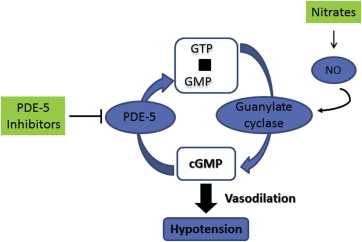
Nitrates are an effective therapy for patients with CAD and chronic AP, but in patients with chronic AP who may be experiencing ED, coadministration of organic nitrates and phosphodiesterase-5 inhibitors contributes to increases in cyclic guanosine monophosphate levels that can lead to the marked vasodilation of blood vessels and unpredictable, symptomatic hypotension ( Figure 2 ). Concomitant use of nitrates and phosphodiesterase-5 inhibitors has also contributed to several cardiac deaths. Therefore, phosphodiesterase-5 inhibitors are contraindicated in the presence of short- and long-acting nitrates. In a study evaluating the effect of the ED drug sildenafil on blood pressure in 16 men aged 45 to 78 years receiving organic nitrates for stable AP, the addition of sildenafil to isosorbide mononitrate resulted in mean maximum reductions in systolic and diastolic blood pressure of −52 and −29 mm Hg, respectively, compared with sildenafil plus placebo (−25 and −15 mm Hg) (p <0.001 vs placebo). Avanafil is not recommended in patients with hypotension at rest (blood pressure <90/50 mm Hg) or hypertension (blood pressure >170/100 mm Hg). Given the combined effect of phosphodiesterase-5 inhibitors and organic nitrates on blood pressure, guidelines from the American Heart Association and the Princeton Consensus Conference recommend that short- and long-acting nitrates be contraindicated in patients with CAD and ED who experience chronic AP with physical activity, including coital AP, if those patients are being treated with phosphodiesterase-5 inhibitors. Patients with CAD who wish to receive phosphodiesterase-5 inhibitor therapy for ED must cease nitrate use and pursue alternative antianginal treatment options. At least 24 hours must pass in patients taking short-acting phosphodiesterase-5 inhibitors such as sildenafil or vardenafil (4-hour half-life) before nitrates can be administered safely; ≥48 hours are needed if patients have been taking long-acting phosphodiesterase-5 inhibitors such as tadalafil (17-hour half-life) before nitrates can be taken.