Biomarkers, particularly natriuretic peptides (NP), complement clinical assessment in patients with heart failure (HF) and may serve as a target level to aid titration of treatment. NP levels that decrease with treatment for acute HF may identify patients at lower risk, but irrespective of the decrease, higher levels at discharge still portend worse outcomes. Beyond NPs, other biomarkers including ST2 have been shown to provide incremental value for prognosis. Although presentation ST2 values are prognostic, admission to discharge change in ST2 and the final ST2 concentration both independently predict patient outcomes in a stronger fashion. Although prognostic thresholds in the hospitalized patient are considerably higher than those used in the office-based setting, a minimum ST2 value of 35 ng/ml is a reasonable starting point for prognosis, noting that many patients will have considerably higher value than this value; as with the NPs, a decreasing value by discharge is desirable, and lower is always better. In conclusion, ST2 values are complementary to NP concentrations, and one can make a good case for serial testing of both biomarkers in the acutely hospitalized patient with HF.
Acute heart failure (AHF) accounts for more than 1,100,000 admissions and 60,000 deaths per year worldwide at a cost of >$39 billion per year. Hospitalization-related charges account for more than half of this cost. Despite advances in therapy, outcomes after hospitalization remain poor. Although the mortality rate has decreased slightly over the last 20 years, the 30-day readmission rate has steadily risen. Nowadays, approximately 1 in 4 patients hospitalized for HF is readmitted within 30 days. Aside from increased cost, HF readmission is also associated with worse prognosis with the risk of death increasing after each readmission. Patients with ≥4 admissions for AHF have >40% mortality risk at 6 months and a median survival of 0.6 months after the readmission. A substantial proportion of these admissions may be avoidable. This concept is supported by the wide variation in 30-day readmission rates between comparable hospitals. Given the high costs of short-term readmission in terms of morbidity and dollars, reducing preventable readmissions within 30 days is now a metric for quality of care by payers in many markets ; this effort has become a major focus of hospitals and health care systems.
Understanding factors leading to early postdischarge hazard is important to address the problem of recurrent events after AHF. It is clear that incomplete relief of congestion while in the hospital along with an inadequate discharge medical regimen contributes to a large number of readmissions. Indeed, with such a substantial focus put on use of loop diuresis during the early treatment phase of AHF, it stands to further reason that neurohormonal activation and myocardial inflammation or fibrogenesis (which are not likely abated by diuretic treatment alone) may contribute substantially to risk. Because strategies that have included home telemonitoring, home nursing visits, and earlier clinic visits have made only small inroads in the problem, newer, creative strategies should be considered. This includes the use of biomarkers. Biomarkers such as natriuretic peptides (NPs) and ST2 are easily available, inexpensive, and are important surrogates of ventricular structure and function. In addition, these 2 biomarkers change in parallel with response to therapy. This report will explore the rationale for using both NP and ST2 measurement not just for risk stratification at discharge but as part of a “biomonitoring” strategy during hospital treatment as we attempt to insure adequate treatment of AHF.
NPs in AHF
In the last decade, the role of “biomonitoring” in the AHF setting has been greatly expanded with most knowledge focused on the NP, as they are currently the gold standard biomarker for assessing prognosis and guiding therapy in patients with AHF. NP are markers of wall stretch and may be better surrogates for congestion than clinical signs because of the lack of sensitivity and low interrater reliability of examination. Baseline and changes in NP levels are associated with changes in filling pressures, and changes in levels during hospitalization are clearly associated with outcomes, with discharge values and the percent reduction both independently predicting repeat hospitalization or death.
Specifically, when measuring BNP or NT-proBNP serially at the time of hospitalization for AHF, best outcomes are seen when NP levels decrease by >30% in hospital and near the baseline concentrations; such concentrations observed in individual patients when stable and euvolemic represent the “dry NP level” which can serve as a target level to aid titration of treatment. Valle et al showed that treating to a BNP level of <250 pg/ml before discharge was associated with favorable outcomes. Whether treatment should be directed toward a proportional decrease in NP or to lowering below a certain threshold requires further study, but the closer they are to baseline levels before decompensation, the better the outcome. A discharge NP level that has not decreased or is discordant with the clinical picture requires careful consideration including further inpatient observation or diagnostic evaluation; clinical impression and risk defined by NP levels may differ significantly.
Caveats in Using NPs
Although NPs may be useful, caveats apply as there are circumstances that may reduce the accuracy of NPs for congestion, such as obesity or circulatory shock. Furthermore, NPs do not reflect all aspects of myocardial disarray that occur in the setting of AHF; in this regard, use of other biomarkers that provide “orthogonal” prognostic information has been explored. Conceptually, the use of markers beyond the NPs provides opportunity to measure in parallel the various deranged physiologies that occur in AHF, including myocardial injury, fibrosis, and risk for remodeling; in this regard, ST2 is a strong candidate for co-measurement with the NPs.
Caveats in Using NPs
Although NPs may be useful, caveats apply as there are circumstances that may reduce the accuracy of NPs for congestion, such as obesity or circulatory shock. Furthermore, NPs do not reflect all aspects of myocardial disarray that occur in the setting of AHF; in this regard, use of other biomarkers that provide “orthogonal” prognostic information has been explored. Conceptually, the use of markers beyond the NPs provides opportunity to measure in parallel the various deranged physiologies that occur in AHF, including myocardial injury, fibrosis, and risk for remodeling; in this regard, ST2 is a strong candidate for co-measurement with the NPs.
Use of ST2 Levels in the Patients With AHF
ST2, a member of the Toll-like/IL-1 receptor superfamily, is expressed both in a transmembrane (ST2 ligand, ST2L) and in a soluble form (sST2). Elevated concentrations of the circulating isoform, sST2, have been related with a more adverse remodeling and a phenotype prone to fibrosis and hypertrophy by sequestering IL-33 and blocking the cardioprotective actions of IL-33/ST2L interaction.
In the setting of AHF, sST2 levels either at admission or discharge are an important predictor of prognosis. In the Pro-BNP Investigation of Dyspnea in the Emergency Department (PRIDE) study, sST2 concentrations >0.20 ng/ml (measured using a research-use-only assay) significantly predicted 1-year mortality in patients with or without HF (negative predictive value 96%). This prediction was dose dependent and additive to NT-proBNP because patients with elevation of both experienced worse outcome. Recently, using the Presage ST2 assay, the cutoff of 35 ng/ml separated those with a good outcome versus those at high risk of death of readmission. In other analyses from PRIDE, the baseline sST2 was found to be predictive of a more adversely remodeled left ventricle, with greater pulmonary pressures, worse ventricular systolic and diastolic function, and a more decompensated profile, with worse symptoms and more congestion. Additionally, the prognostic importance of sST2 for predicting death in this cohort extended well out to 4 years from presentation.
sST2 Response to Therapy in Patients With AHF
As discussed in the section on laboratory testing elsewhere in this consensus document, sST2 has an index of variability and biologic variation that suggests that it is of even greater value for prognosis when measured sequentially. Studies have supported this suggestion. Additionally, as sST2 values are not significantly affected by age, gender, body mass index, and renal function, it is appealing to consider this biomarker as a prototypical risk marker in AHF. To this end, in an early study, Boisot et al ran daily sST2 concentrations on a group of patients admitted with AHF ( Figure 1 ). The first relevant observation in this study is that concentrations of sST2 are dynamic in the short term after admission, decreasing rapidly after entry to the hospital in those who had uncomplicated short-term follow-up; in contrast, those dying by 6 months had a significant rise after admission.
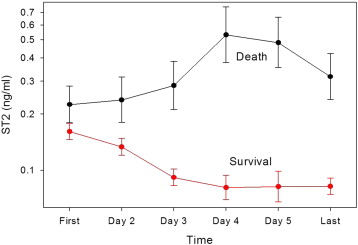
The next published study was by Manzano et al, which measured the newer Presage ST2 assay immediately at presentation in the emergency department and on day 4 of hospitalization. sST2 concentrations decreased from a median of 62 to 44 ng/ml (p <0.001), and those patients with persistently greater concentrations on day 4 had a significantly higher risk of death. In a final study of Breidthardt et al, sST2 values significantly reduced from admission to 48 hours, particularly in those with uncomplicated courses with a median sST2 reduction of 42% in survivors versus 25% in decedents (p <0.01).
In each of the studies mentioned previously, the prognostic information of sST2 was additive (or superior) to NPs and was not affected by renal function or other confounders that often affect biomarkers. In aggregate, these data provide definitive evidence of the dynamic nature of sST2 values in the setting of hospitalized AHF.
sST2 Response as Prognosticator
The second issue is if the response of sST2 concentrations to therapy identifies a different prognosis. As noted, those patients whose sST2 level decreased with treatment had a much better prognosis than those whose sST2 levels did not respond or even increased with treatment. This was the first evidence that patients with HF can be classified as responders or nonresponders on the basis of whether there is significant movement of sST2 levels. Two other recently published reports have demonstrated the efficacy of targeting treatment of HF with sST2 levels : the response to therapy either at 24 hours or 4 days was able to identify a different prognosis. This different prognosis was not only observed in the short-term 90-days but also remained in long-term follow-up at 1 year and 2 years from hospital discharge.
Of note is that the admission to discharge change in sST2 and the final value both make a difference for prognosticating over an extended duration. The combined assessment of baseline ST2 and early ST2 changes (48 hours) provided further prognostic information in excess of baseline ST2 levels and a clinical model. Patients with both repeated measures, admission and on day 4, below the cutoff point had a very low mortality rate (3%), whereas those with both sST2 values above the cutoff had the highest mortality rate (50%) ( Figure 2 ), and the combined assessment of repeated measures improved risk stratification over NP and clinical variables. In other words, not only a response is important but also the higher the response and the lower the level at discharge, the better the result.




