, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Atrial septal defects (ASD) are seen in 1 per 1,500 live births and account for 30–40 % of all adult congenital heart disease [1]. There are five basic types of atrial septal defects (Fig. 11.1): (1) secundum ASD, (2) primum ASD, (3) sinus venosus ASD (superior and inferior types), (4) coronary sinus ASD, and (5) common atrium (simultaneous combination of two or more types of atrial septal defect) [2]. See Table 11.1.
11.1 Atrial Septal Defects
Atrial septal defects (ASD) are seen in 1 per 1,500 live births and account for 30–40 % of all adult congenital heart disease [1]. There are five basic types of atrial septal defects (Fig. 11.1): (1) secundum ASD, (2) primum ASD, (3) sinus venosus ASD (superior and inferior types), (4) coronary sinus ASD, and (5) common atrium (simultaneous combination of two or more types of atrial septal defect) [2]. See Table 11.1.
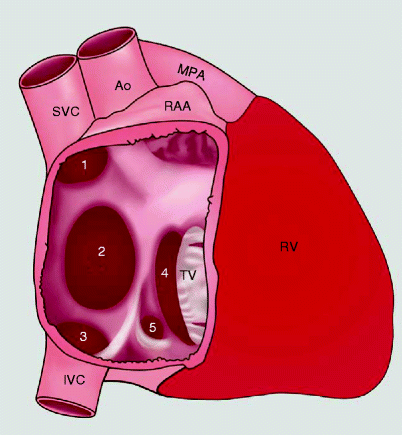

Fig. 11.1
The variants of atrial septal defects: (1) superior sinus venosus defect, (2) secundum defect, (3) inferior sinus venosus defect, (4) primum defect, and (5) coronary sinus defect. Ao aorta, MPA main pulmonary artery, IVC inferior vena cava, RAA right atrial appendage, RV right ventricle, SVC superior vena cava, TV tricuspid valve
Table 11.1
Types of ASD
Secundum |
Primum |
Sinus venosus (superior and inferior type) |
Unroofed coronary sinus |
Common atrium |
As discussed in depth in Chap. 1, the normal septum develops via apposition of the septum primum, a thin membrane arising from the roof of the primitive atrium, and the septum secundum, a membrane arising from the ventrocranial wall of the primitive atrium. A defect in the central portion of the septum secundum, the foramen ovale, is normally closed by infolding of the atrial septa, forming an indentation referred to as the fossa ovalis. Defects within the area of the fossa ovale are known as secundum atrial septal defects. If there is an extensive deficiency of the atrial septum closure, the ASD can extend beyond the fossa ovale. Extension may occur superiorly or posteroinferiorly to the origin of the superior and inferior vena cava, respectively (sinus venosus ASD), inferiorly to the atrioventricular junction (primum ASD), or posteriorly to the coronary sinus (coronary sinus ASD).
11.1.1 Secundum (Fossa Ovale) ASD
This is the most common type of ASD in the general population and accounts for 70 % of ASDs and 6–10 % of all congenital heart disease [2]. The secundum ASD is usually an isolated lesion, but it can be seen in association with other forms of congenital heart disease. The two common associations are mitral stenosis (Lutembacher syndrome) and absent radius (Holt–Oram syndrome). Secundum ASD is also associated with mitral valve prolapse.
Figures 11.2, 11.3, 11.4, 11.5, and 11.6 are representative illustrations of the spectrum of secundum ASD.
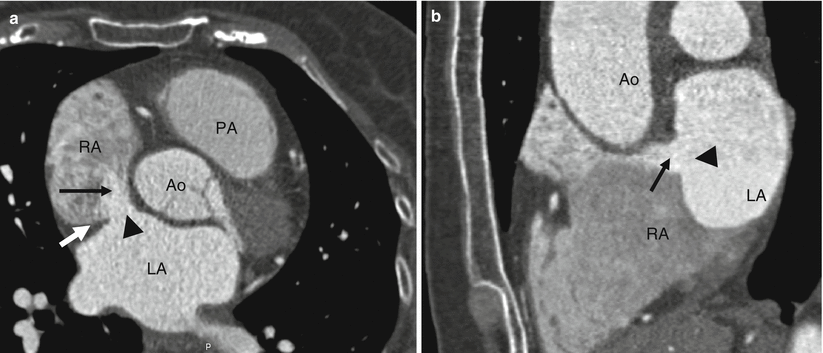
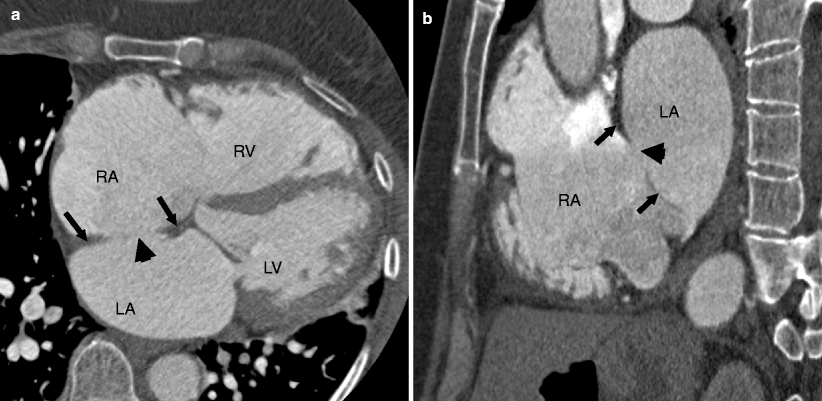
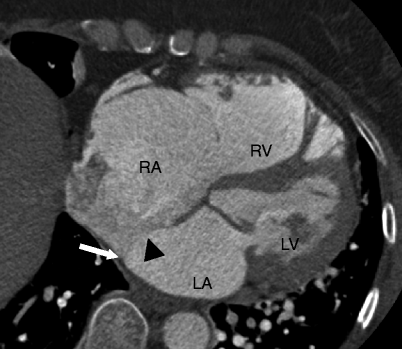
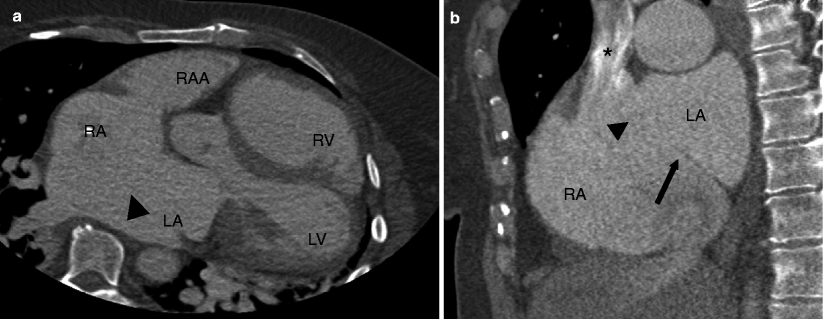
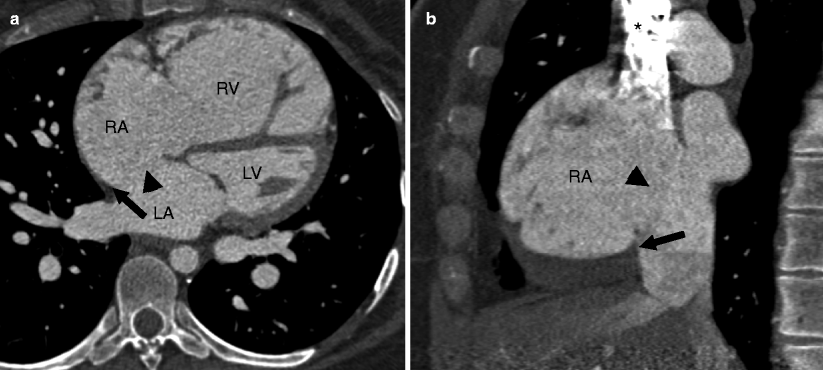

Fig. 11.2
Panels (a) and (b) are small secundum atrial septal defects (ASD) (black arrowheads) limited entirely to the fossa ovale. The ASD in panel (a) demonstrates a good tissue rim thickness (white arrow). Note the jets of contrast (black arrows) entering the right atrium from the left atrium. The right atrial size is normal indicating the absence of volume overload. RA right atrium, PA pulmonary artery, Ao aorta, LA left atrium

Fig. 11.3
Panels (a) and (b) are large secundum atrial septal defects (ASD) (black arrowheads) associated with right atrial (panels a and b) and right ventricular enlargement (panel a), indicating volume overload. Note a good tissue rim, exceeding 3 mm in all directions (black arrows) in both panels. LA left atrium, LV left ventricle, RA right atrium, RV right ventricle

Fig. 11.4
A large secundum atrial septal defect (ASD) (black arrowhead) with a posteroinferior rim deficiency (white arrow). RA right atrium, RV right ventricle, LA left atrium, LV left ventricle

Fig. 11.5
Panels (a) and (b) illustrate a CT image of a large secundum atrial septal defect (ASD) (black arrowhead). Panel (a) (axial plane) suggests that the ASD lacks both anterosuperior and posterosuperior rims and thus appears as one common atrium in this view. However, the sagittal view (panel b) demonstrates the presence of only a posteroinferior rim (black arrow). RA right atrium, RV right ventricle, LA left atrium, LV left ventricle, Asterisk superior vena cava, RAA right atrial appendage, LAA left atrial appendage

Fig. 11.6
Panel (a) is a large secundum atrial septal defect (ASD) (black arrowhead) with the absence of an anterosuperior rim (black arrow). Panel (b) is a large secundum ASD (black arrowhead) lacking a posteroinferior rim (black arrow). RA right atrium, RV right ventricle, LA left atrium, LV left ventricle, Asterisk superior vena cava
11.1.2 Primum ASD
Primum atrial septal defects (Fig. 11.7), also called atrioventricular septal defects, account for 15–20 % of ASDs [2]. This ASD presumably results from failure of the endocardial cushion to close the ostium primum and occurs between the fossa ovalis and the level of the atrioventricular valves. Primum ASDs are often associated with atrioventricular valve abnormalities (cleft mitral valve) and with trisomy 21 and heterotaxy syndromes. Unrepaired primum ASDs are very rare in adults [3] since correction is usually necessary prior to adulthood.
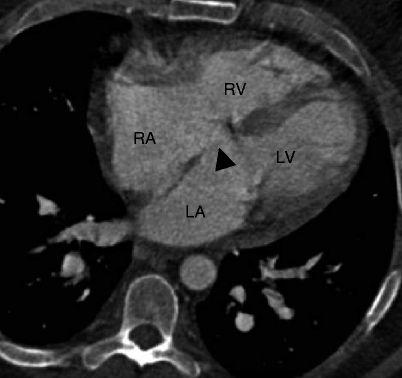

Fig. 11.7
Primum atrial septal defect (ASD). An axial CT shows the ASD (black arrowhead) located between the level of the fossa ovalis and atrioventricular valves. This defect occurs in the lower part of the atrial septum close to the ventricular inlet valves. RA right atrium, RV right ventricle, LA left atrium, LV left ventricle
11.1.3 Sinus Venosus ASD
A superior sinus venosus atrial septal defect occurs at the junction of the superior vena cava (SVC) and right atrium and accounts for 5–10 % of all ASDs [2]. It often coexists with partial anomalous pulmonary venous return, usually from the right upper lobe to the SVC near its junction with the right atrium. Sinus venosus ASDs are associated with an increased incidence of pulmonary hypertension due to increased flow to the lungs from the anomalous pulmonary venous return. An inferior sinus venosus ASD is much less common than a superior sinus defect and occurs at the junction of the inferior vena cava (IVC) and right atrium.
Treatment of sinus venosus ASDS is surgical closure and reimplantation of the anomalous pulmonary veins. Alternatively, a baffle may be created to correct return of the anomalous pulmonary venous flow back to the left atrium [2]. Rarely, the superior vena cava can enter the right atrium via an accessory vein.
Figures 11.8, 11.9, 11.10, 11.11, and 11.12 are examples of various types of sinus venosus ASD.
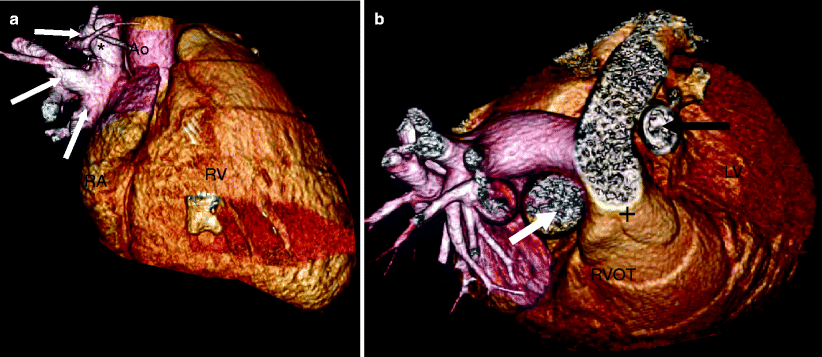
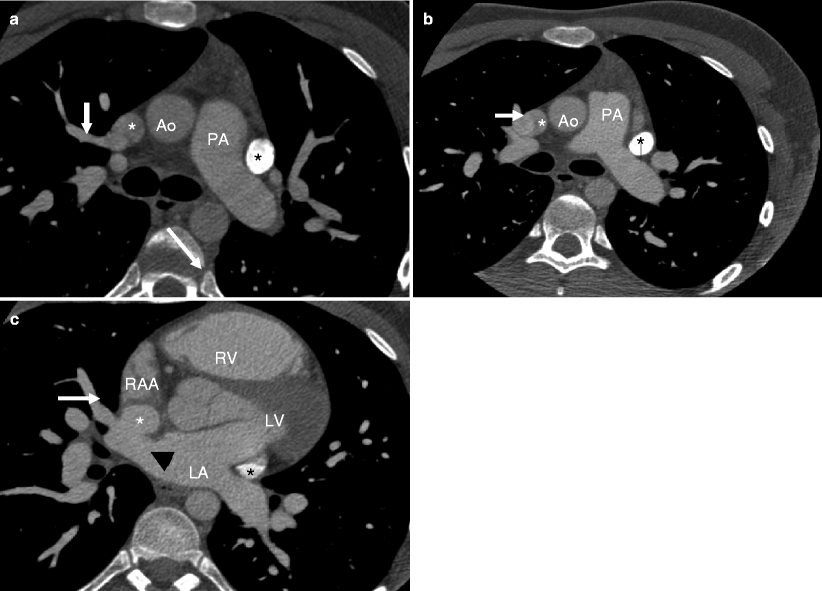
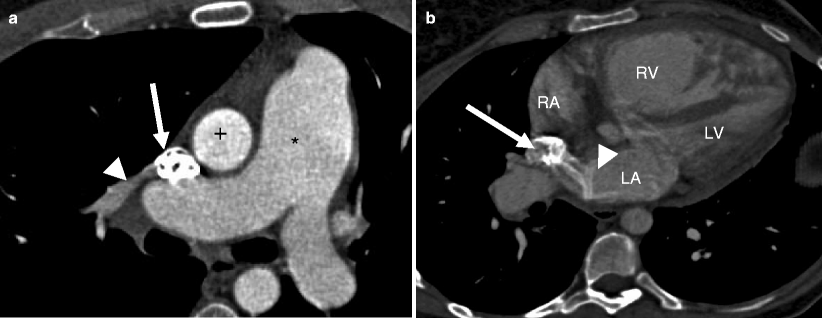
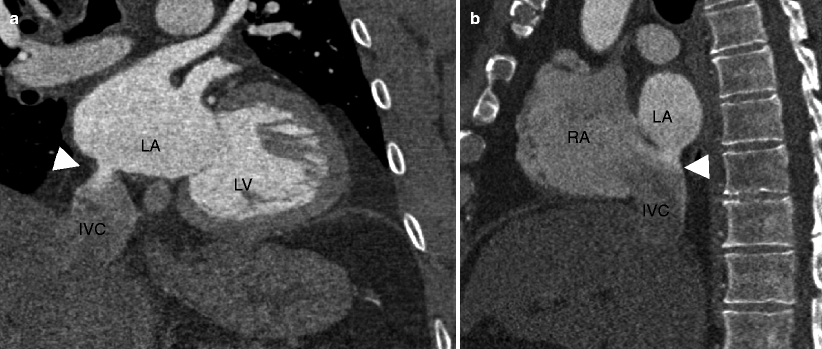
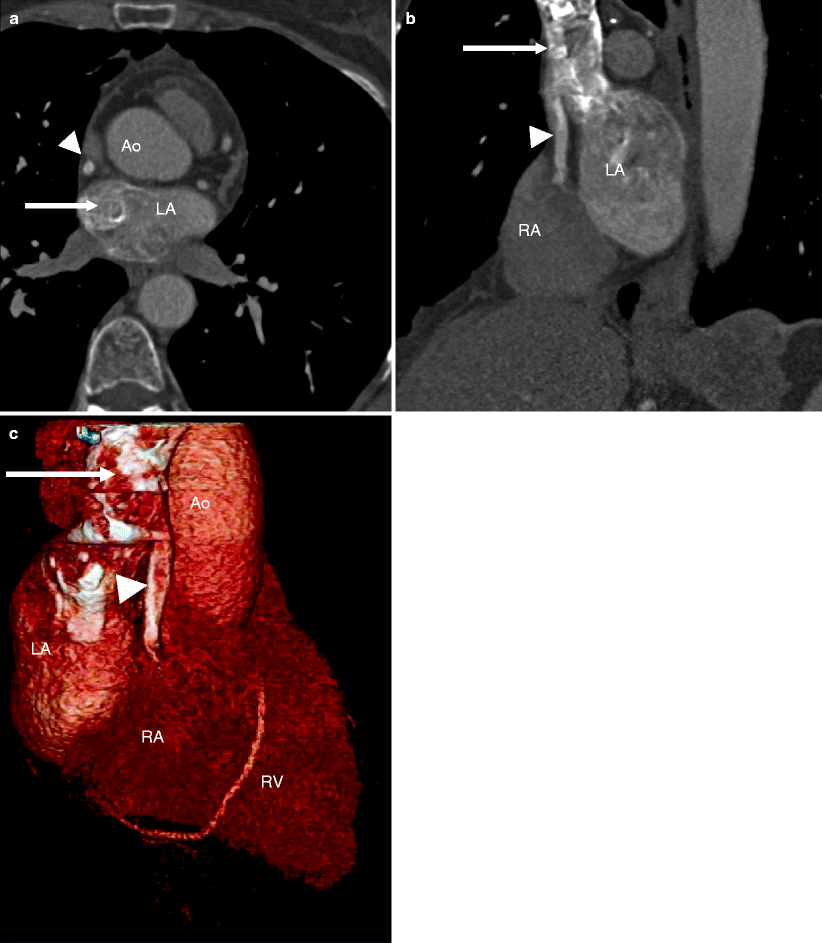

Fig. 11.8
Panels (a) and (b) are 3D volume-rendered computed tomography images from a patient with a sinus venosus atrial septal defect. Panel (a) demonstrates partial anomalous pulmonary venous return (three right-sided pulmonary veins (white arrows) entering the superior vena cava, SVC). Panel (b) shows a left-sided SVC (black arrow). In this panel the normal SVC is depicted by the white arrow. RVOT right ventricular outflow tract. Plus sign (+): pulmonary artery. RA right atrium, RV right ventricle

Fig. 11.9
Maximum intensity projections of the sinus venosus atrial septal defect from the patient in Fig. 11.8. Here panels (a), (b), and (c) are axial views each showing 1 of the 3 anomalous pulmonary veins (black arrows, white arrows) entering the superior vena cava (asterisk). Panel (c) illustrates the SVC straddling both atria (black arrowhead). The persistent left-sided SVC is again noted by the black asterisk. White asterisk superior vena cava, Ao aorta, PA pulmonary artery, RAA right atrial appendage, RV right ventricle, LA left atrium, LV left ventricle

Fig. 11.10
A superior sinus venosus atrial septal defect (ASD). Panel (a) is an axial scan demonstrating an anomalous vein (white arrowhead) from the right upper lobe entering the superior vena cava (SVC, white arrow). Note the enlarged pulmonary arteries resulting from pulmonary hypertension (black asterisk). Panel (b) is an axial view at a more inferior plane illustrating the actual ASD with a jet of contrast (white arrowhead) flowing from the SVC (white arrow) to the left atrium (LA). Typically the flow into the left atrium is along the lateral wall, as in this case. RA right atrium, RV right ventricle, LA left atrium, LV left ventricle, Plus sign (+) ascending aorta

Fig. 11.11
An example of an inferior sinus venosus ASD. Panels (a) and (b) are multiplanar reformatted images and depict a contrast-filled communication (panel a, white arrowhead) between the inferior vena cava (IVC) and left atrium (LA) with the IVC straddling both atria (panel b, white arrowhead). LV left ventricle, RA right atrium

Fig. 11.12
An unusual case of sinus venosus atrial septal defect (ASD). Panel (a) is an axial reformat, panel (b) is an oblique reformat, and panel (c) is a 3D volume-rendered image. All images show the superior vena cava (SVC, white arrow) entering the left atrium (LA) while an accessory vein (white arrowheads) enters the right atrium (RA). LA left atrium, Ao aorta, RV right ventricle
11.1.4 Unroofed Coronary Sinus ASD
Unroofed coronary sinus ASD is rare, comprising less than 1 % of all atrial septal defects [2]. It is thought to result from a failure of separation of the superior wall of the coronary sinus with the left atrium, leading to a direct communication between the coronary sinus and the left atrium. It is usually associated with a persistent left superior vena cava (LSVC). Persistent LSVC occurs in 0.1–0.5 % of the general population and 8 % of anomalies drain into the left atrium [4]. An unroofed coronary sinus ASD is seen in 75 % of patients with persistent LSVC that drains into the left atrium [4]. Unroofed coronary sinus is classified into four groups. Type I is a completely unroofed coronary sinus with persistent LSVC. Type II is a completely unroofed coronary sinus without persistent LSVC. Type III is a partially unroofed midportion coronary sinus defect. Type IV is a partially unroofed terminal portion coronary sinus defect. The fenestration from the coronary sinus into the left atrium typically occurs between the left atrial appendage and the left upper pulmonary vein [4].
Figure 11.13 is an example of an unroofed coronary sinus type ASD.
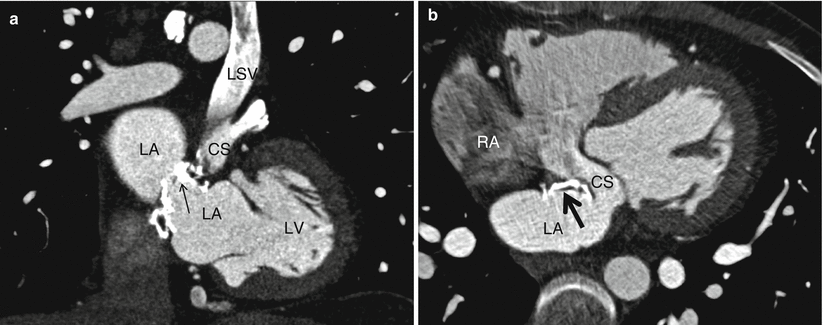

Fig. 11.13
Represents an unroofed coronary sinus atrial septal defect in a 33 year old patient with repair in childhood who now presents with right sided heart failure and evidence of an intracardiac shunt. Panel (a) demonstrates a persistent left sided superior vena cava entering the coronary sinus. A calcified septal closure patch which does not cover the entire defect is also demonstrted (arrow). Panel (b) demonstrates an intra-atrial communication via the unroofed coronary sinus. LA left atrium, RA right atrium, LSV persistent left sided superior vena cava, CS coronary sinus, LV left ventricle
11.1.5 Common Atrium
Common atrium (confluence of two or more types of ASD defects) is characterized by complete absence of atrial septum and occurs in association with other complex defects, frequently heterotaxy syndrome. It is very rarely seen in the adult population [5].
Figure 11.14 depicts a common atrium.
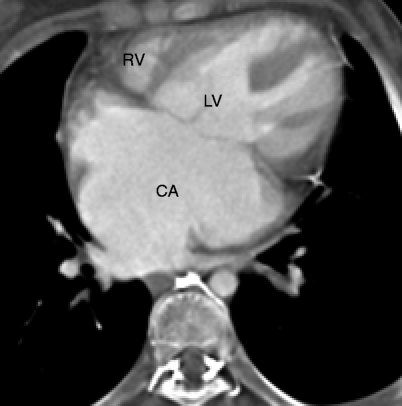

Fig. 11.14
A depiction of a common atrium. This transverse view shows one common atrial chamber (CA) with complete absence of the atrial septum. RV right ventricle, LV left ventricle
11.1.6 Patent Foramen Ovale
Patent foramen ovale (PFO) is the most common form of interatrial communication. It is caused by a failure of fusion of the flap valve of the fossa ovalis. The prevalence of PFO declines progressively with age (34 % up to age 30 years, 25 % for age 30–80 years, and 20 % older than 80 years) [7]. Two types of PFO exist. The first is the incompetent valve type and results in right-to-left shunting only when right atrial pressure exceeds that of the left atrium such as during a Valsalva maneuver. The second type is called the stretched type and is due to high left atrial pressure (such as seen in left heart failure) which stretches the fossa ovalis flap valve to the point of incompetence, resulting in a left-to-right shunt. While a single defect is most common in PFO, multiple fenestrations may also be seen.
Figure 11.15 is an example of a PFO seen on CT.
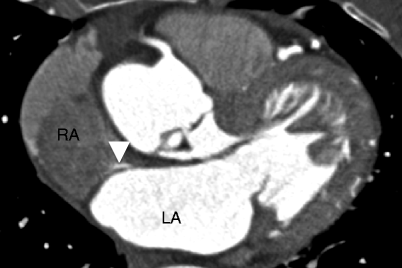

Fig. 11.15
Patent foramen ovale. An axial scan shows a thin contrast jet (white arrowhead) that is directed parallel to the intra-atrial septum. Typically, the flow is along the anterior part of the atrium and parallels the atrial septum. LA left atrium, RA right atrium
Atrial septal aneurysm (ASA) is associated with a PFO in 30 % of the cases [8]. ASA is defined as an abnormal bulging of the interatrial septum with an excursion of at least 10 mm and a base span of at least 15 mm [8]. This is thought to be due to redundancy of the valve of the fossa ovalis and/or excessive mobility of the atrial septum with ballooning into the right or left atrial chamber. ASA may be associated with mitral valve prolapse and atrial arrhythmias.
Figure 11.16 demonstrates an atrial septal aneurysm.
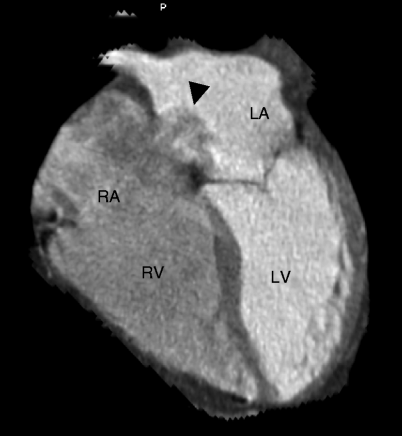

Fig. 11.16
Atrial septal aneurysm (ASA, black arrow). This four-chamber view shows the bulging of the atrial septum with a base span >15 mm. The excursion is>10 mm. There is no contrast jet across the atrial septum in this aneurysm. Note the clear demarcation between the contrast-filled left atrium (LA) and the relatively contrast-poor right atrium (RA). ASA can mimic a left-to-right or bidirectional shunt due to excessive motion of very floppy interatrial septum. RV right ventricle, LV left ventricle
11.1.7 Clinical Findings of Atrial Septal Defects
Clinical findings of isolated atrial septal defects are usually related to left-to-right shunting. The degree of shunting is determined by the size of the defect and the relative compliance of the right and left ventricles. Secundum ASDs that have a sufficient rim of tissue around the septal defect can be closed percutaneously with septal occluder devices, most commonly the AMPLATZER septal occluder device. All other hemodynamically significant ASDs require surgical closure.
11.1.8 Cardiac Computed Tomography (CT) in the evaluation of ASD
Echocardiography is the method of choice to diagnose ASD but CT can be used when echocardiography is indeterminate [8–12]. The CT diagnosis of ASD is based on identifying a defect in the septal tissue and/or the presence of increased contrast opacification in the right heart despite proper timing of the scan after contrast bolus administration. The typical appearance of an ASD on CT is that of a contrast jet from the left atrium to the right atrium. Other findings of a left-to- right shunt include right ventricular and right atrial enlargement. Secundum ASD can be differentiated from a patent foramen ovale by the appearance of the jet. A PFO has a very thin, channel-like appearance and the contrast jet is at a slight angle or parallel to the interatrial septum or atrial roof as it courses from the left atrium to the right atrium (Fig. 11.15) [13, 14]. The secundum and sinus venous defects are broader and the former flows into the central part of the left atrium, while the latter flows along the lateral wall (Figs. 11.2, 11.3, 11.4, 11.5, 11.6, 11.8, 11.9, 11.10, 11.11, and 11.12) [14, 15].
Associated findings of partial anomalous pulmonary venous return may be noted in sinus venosus-type ASDs (Figs. 11.8, 11.9, 11.10, 11.11, and 11.12). With primum ASDs, an associated cleft mitral valve or associated ventricular septal defects may be noted.
Morphological changes of pulmonary hypertension found in long-standing larger ASDs include pulmonary arterial and right heart chamber enlargement.
CT can provide accurate anatomic assessment prior to percutaneous closure of an ASD or PFO. The necessary measurements include an assessment of the long- and short-axis views of the defect and measurement of the area of the defect (Fig. 11.17) In addition, the thickness of the rim of surrounding tissue is important to note. Measurements are made in end systole.
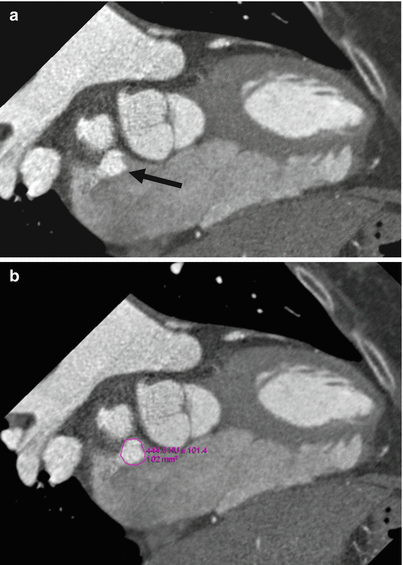

Fig. 11.17




Measurement of the atrial septal defect (ASD) area. Oblique en face views allow measurement of defect dimension and area. CT images should be reconstructed so that the maximum size of the defect in long and short axis can be visualized and measured with electronic calipers at the workstation. Usually, an oblique reconstruction with en face view of the ASD suffices. Measurements are made at end systole to obtain the greatest size of the ASD. The area is automatically calculated by tracing the electronic calipers along the circumference of the ASD. Circle Traced area of the ASD, black arrow ASD
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


