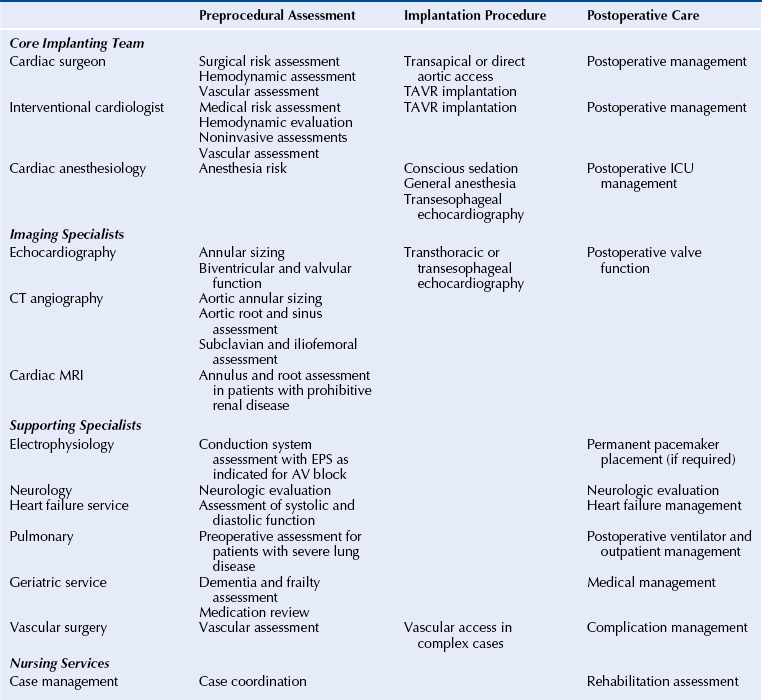
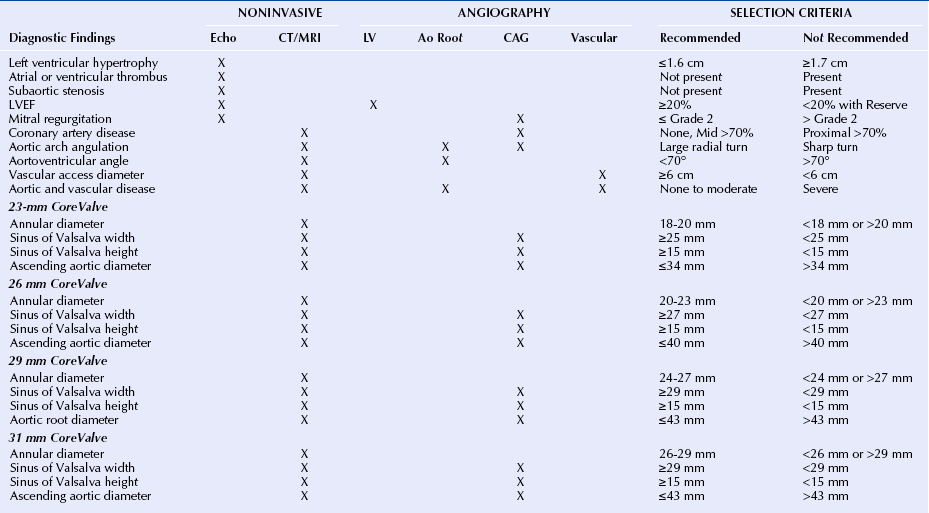
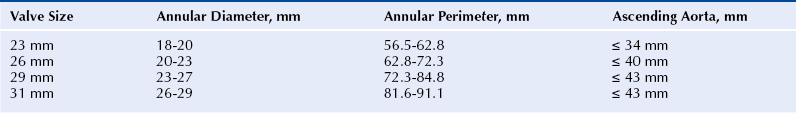
Chapter 7 • Transcatheter aortic valve replacement (TAVR) using the self-expanding Medtronic CoreValve Revalving System (Medtronic, Maple Grove, Minn.) transcatheter heart valve (THV) provides an alternative to surgical aortic valve replacement (SAVR) in patients who are poor candidates for surgery. • Unique features of the CoreValve design are its ability to conform to the eccentric annulus, a supraannular location of the low-profile porcine pericardial valve with a long commissural length to reduce stress, a constrained region of the nitinol frame to access the coronary arteries, and an outflow region that orients the valve to provide maximal flow. • Preprocedural evaluation using echocardiography and multidetector computed tomography (MDCT) imaging allows patient selection and optimal sizing for the CoreValve THV. Patients who have unsuitable iliofemoral anatomy can be treated by means of a subclavian or direct aortic approach. • Optimal CoreValve implantation technique includes positioning of the CoreValve frame less than 6 mm below the aortic annulus; evaluation for postimplantation paravalvular regurgitation using echocardiography, hemodynamics, and aortography; and monitoring for conduction system abnormalities. • Complications of TAVR include stroke, conduction system abnormalities, paravalvular regurgitation, and vascular complications and may be minimized with comprehensive preprocedural planning and optimal implantation technique. Aortic stenosis is the most common valve disorder in developed countries.1 The recommended therapy for the majority of patients with symptomatic aortic stenosis is SAVR.1,2 Surgical risk assessment is most often obtained through a thorough review of the patient’s clinical history and physical examination, aided by use of the Society for Thoracic Surgery Predicted Risk of Mortality (STS-PROM) score estimated 30-day mortality rate3 or other scores that provide a quantitative assessment of outcome. A number of clinical and anatomic factors are considered by the surgical team that may render the patient an unsuitable candidate for SAVR, including aortic calcification4,5 (Figure 7–1, A-F), hostile mediastinum related to the crossing of the left internal mammary artery (LIMA) across the midline of the chest (Figure 7–1, G), severe lung or liver disease, frailty, and renal failure.6–10 In patients who are deemed unsuitable or “high risk” for sAVR because of underlying comorbidities, TAVR has been used as an alternative for symptom relief and extension of life.11–15 Sustained improvement after CoreValve TAVR three years after implantation has been reported.16 Figure 7–1 Porcelain aorta and hostile mediastinum. The 18F Medtronic CoreValve ReValving System is a THV comprised of a self-expanding nitinol support frame that anchors a trileaflet porcine pericardial tissue valve, an 18F delivery catheter, and a valve loading system (Figure 7–2). The nitinol frame was designed with three levels of radial and hoop strength. The frame inflow exerts high radial expansive force to secure the frame within the aortic annular location, allowing the frame to partially conform to the noncircular shape of the aortic annulus. The “constrained” center portion of the frame has high hoop strength that resists compression and shape deformation, which is critical as this portion of the frame contains the “supraannular” valve porcine leaflets. The constrained portion of the frame is concave to avoid the coronary ostia, allowing coronary artery cannulation after CoreValve implantation. The outflow portion of the frame serves to orent the frame to the aorta parallel to flow through the valve. Figure 7–2 CoreValve ReValving System. The porcine pericardium was selected because of its lower profile (compared with bovine pericardium) and for its long-term durability. The zigs (or cells) of the frame are 8 mm in length and are connected in their central portion (Figure 7–3). When the frame is constrained in its delivery sheath, the joint distances are separated by 4 mm, providing radiopacity to aid in the positioning of the device as the constraining membrane of the delivery sheath is withdrawn. Optimal positioning for the CoreValve THV is at a depth of 4 to 6 mm below the aortic annulus. The CoreValve THV is available in 23-mm (for use with aortic annular diameters between 18 and 20 mm), 26-mm (annular diameters between 20 and 23 mm), 29-mm (annular diameters between 24 and 27 mm), and 31-mm (annular diameters between 26 and 29 mm) sizes (Figure 7–4). Figure 7–3 Magnified view of the CoreValve frame. A collaborative and multidisciplinary approach to the evaluation and management of patients with complex aortic valve disease has led to the crucial development of the “heart team.” (Table 7–1).17,18 The heart team is composed of cardiac surgeons, interventional cardiologists, cardiac anesthesiologists, imaging specialists, electrophysiologists, neurologists, heart failure cardiologists, and geriatricians.19 Vascular surgeons may be needed for complex access and management of vascular complications after the procedure. Nursing services provide critical support services in the preoperative and postoperative periods, as well as coordination of outpatient care after TAVR. The primary purpose of the heart team is to coordinate the risk assessment of the patients and to facilitate the preoperative and postoperative care. The primary tool for the assessment of 30-day cardiac surgical mortality in the United States is the STS-PROM score.3 Although the STS-PROM score remains a key component in assessing risk for sAVR, it is supplemented by additional factors affecting outcomes after surgery that are not included in the STS-PROM. Additional risk factors termed STS Plus are being used as part of the CoreValve US Pivotal Trial and take into account factors such as degree of aortic calcification, pulmonary hypertension, liver disease, chest deformity, hostile mediastinum, severe chronic obstructive pulmonary disease, home oxygen or continuous positive airway pressure (CPAP), and markers of frailty (body mass index [BMI] <21, albumin <3.3, wheelchair bound, and not living independently). The importance of the heart-team approach has been endorsed by several recent multispecialty guidelines.20,21 Training standards for the structural heart disease heart team have been established.20,21 Patients enrolled in regulatory trials in the United States that have evaluated the CoreValve THV and the Edwards SAPIEN balloon-expandable THV (Edwards Lifesciences, Irvine, Calif.) have met strict risk-assessment criteria for enrollment.11,12 All patients were required to be symptomatic from their aortic stenosis and have New York Heart Association class II or greater heart failure symptoms. “Inoperable” (or “extreme risk”) patients are those who have been assessed by two cardiac surgeons and are deemed to have greater than a 50% risk of mortality or irreversible morbidity within 30 days after sAVR.11 “High-risk” patients are those deemed to be at substantial risk for sAVR based on an estimated 30-day mortality rate of 15% or more.12 This has generally been established as an STS-PROM score of 8 or higher and consideration for other factors that contribute to surgical mortality. Echocardiographic criteria for enrollment in clinical trials in the United States have included an aortic valve area of 0.8 cm2 or less (or index ≤0.5 cm2) associated with a mean gradient greater than 40 mmHg or peak gradient greater than 4 m/sec shown with at-rest echocardiography. In patients with reduced left ventricular function, these measurements can be augmented by dobutamine infusion at the time of the echocardiogram or simultaneous measurement during cardiac catheterization.11,12 These criteria have been liberalized for CoreValve commercial use outside the United States (aortic valve area < 1.0 cm2 and no minimal gradients or jet velocities). Patients with severely reduced left ventricular ejection fraction (LVEF <20%); outflow tract gradients caused by basal septal hypertrophy; severe mitral regurgitation; low-gradient, low output aortic stenosis without contractile reserve; and bioprosthetic valve failure were excluded from the clinical trials in the United States.11,12 Expanded clinical use outside the United States with the CoreValve THV has also included a number of clinical subsets, including patients with bioprosthetic valve failure,22,23 reduced left ventricular function,24 and aortic regurgitation,25 as well as those with low-gradient, low flow aortic stenosis,26,27 severe mitral regugutation,28 bicuspid valves,29 and underlying coronary artery disease.30 The design of the CoreValve THV requires that there is careful evaluation of the aortic valvar complex with imaging studies before the procedure, most often with MDCT (Table 7–2). Accurate and reliable aortic annular measurements are key to determining the size of device used, and also in reducing the impact of paravalvular regurgitation (Figure 7–5).31 Owing to the eccentric geometry of the aortic annulus, correlative studies have shown a systematic underestimation of the annular size by two-dimensional echocardiography alone.32–34 MDCT provides a better estimate of the long- and short-axis diameters of the aortic annulus, surface area, and perimeter measurements,32,35,36 and has been used extensively for annular sizing in the United States for the CoreValve THV. Oversizing of the THV and excessive calcification may result in aortic annular rupture.37,38 Three-dimensional imaging using transesophageal echocardiography may provide accurate assessments of the aortic annulus.39 Figure 7–5 Multidetector computed tomography assessment of annular sizing. Annular diameters outside the range of smaller than 18 mm or larger than 29 mm cannot be treated with the current generation of the CoreValve device (Table 7–3). Adequate sinus of Valsalva width (>25 mm for the 23-mm valve; >27 mm for the 26-mm valve; and >29 mm for the 29-mm and 31-mm valves) (Figure 7–6) and Sinus of Valsalva height (>15 mm) is required to avoid coronary occlusion. Determination of the height of the coronary arteries is important to understand the potential displacement of the leaflets during CoreValve THV deployment (Figure 7–7). The ascending aortic diameter should be less than 40 mm for the 26-mm valve and less than 43 mm for the 29-mm and 31-mm valves (Figure 7–8). Other anatomic criteria include an adequate aortoventricular angle smaller than 70 degrees for the iliofemoral and left subclavian access routes and smaller than 30 degrees for the right subclavian approach (Figure 7–9), and the estimation of the extent and distribution of annular calcification may be reliably assessed (Figure 7–10). Dense calcification of the left coronary sinus, aortomitral curtain, and mitral valvular calcification should be avoided because of the potential for the inability to fully expand the frame (Figure 7–11). Figure 7–6 Multidetector computed tomography measurements of the aorta valvar complex. Figure 7–7 Location of the coronary arteries. Figure 7–8 Tubular aorta. Figure 7–9 Determination of the aortoventricular angle. Figure 7–10 Assessment of aortic valve calcification. Figure 7–11 Mitral annular calcification. The 18F CoreValve Revalving System is advanced through an 18F sheath, requiring that the access vessel diameter is 6 mm or greater in a noncalcified vessel and 7 mm or greater for a severely calcified vessel (Figure 7–12). Although initial studies used aortography to select patients for CoreValve placement, more recent screening procedures have included MDCT of the abdomen and pelvis, including the ascending and descending aorta and peripheral run-off vessels (subclavian, iliac, and femoral arteries).40 Figure 7–12 Multidetector computed tomography peripheral vascular assessment.
Self-Expanding Transcatheter Aortic Valve Replacement Using the CoreValve Transcatheter Heart Valve
7.1 Key Points
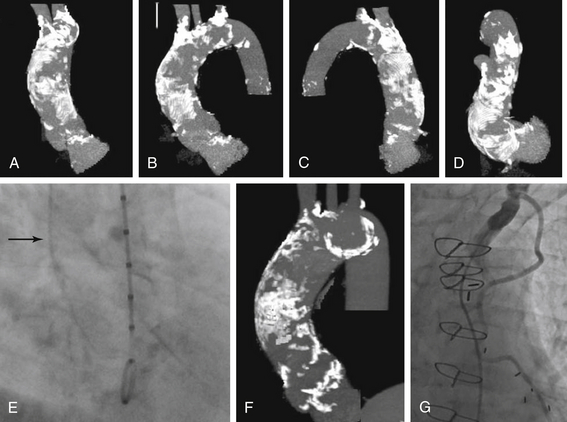
A-D, Rotational CT of the aortic root demonstrates diffuse calcification of the ascending aorta precluding cross-clamping during surgical aortic valve replacement. E, Cineangiography of the aortic root during cardiac catheterization reveals dense calcification of the aortic root (arrow). F, Another example of a densely calcified aorta is shown. G, A left internal mammary artery that transverses beneath the sternum renders the patient at prohibitive risk for repeat surgery.
7.2 CoreValve ReValving System
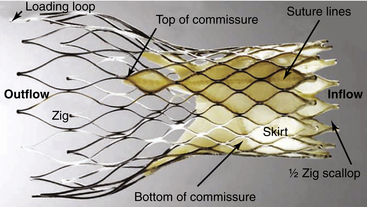
The CoreValve transcatheter heart valve frame is comprised of a series of staggered nitinol zigs (or cells) that are 8 mm in length at the level of the inflow. The inflow portion of the frame provides radial expansive strength at the annulus to secure its location within the left ventricular outflow tract and aortic annulus. There is a 12-mm skirt that is constructed of porcine pericardium that prevents leaking around the valve frame. The central (or constrained) portion of the frame contains the trileaflet porcine pericardial valve and allows access to the coronary arteries. The leaflet commissures are longer than standard surgical valves to distribute the diastolic load along the length of the valve rather than at its posts. (Reproduced from Michiels, R. CoreValve ReValving system for percutaneous aortic valve replacement. In Serruys PW, Piazza N, Cribier A, et al., editors. Transcatheter aortic valve implantation: tips and tricks to avoid failure, New York: Informa Healthcase, 2010, with permission).
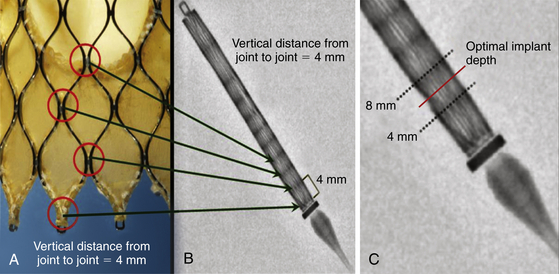
A, The zigs (or cells) at the inflow region are 8 mm in length and staggered with a vertical distance from joint to joint of 4 mm. B, Constrained within the delivery sheath, there are radiopaque lines every 4 mm. C, The radiopaque marker at the distal tip of the retaining membrane provides guidance for optimal implantation 4 to 6 mm below the noncoronary sinus of Valsalva.
7.3 The Heart Team
Patient Selection
Anatomic Criteria
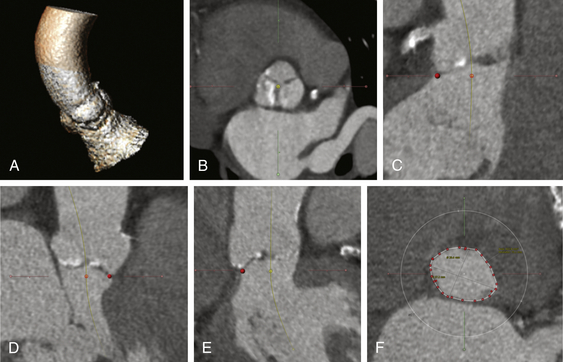
A, The aorta valvar complex is constructed by identification of a centerline that begins in the left ventricular outflow tract through the aortic annulus and extending into the ascending aorta. B, A cross-section of the sinus of Valsalva is obtained from the centerline. C, The basal locations of the left coronary sinus, right coronary sinus (D), and noncoronary sinus (F) are identified (dots).
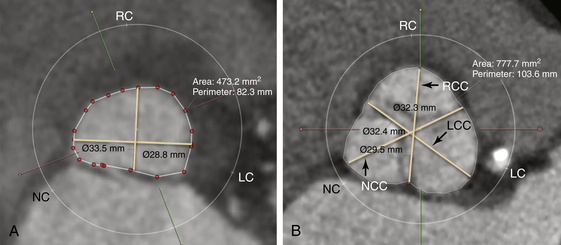
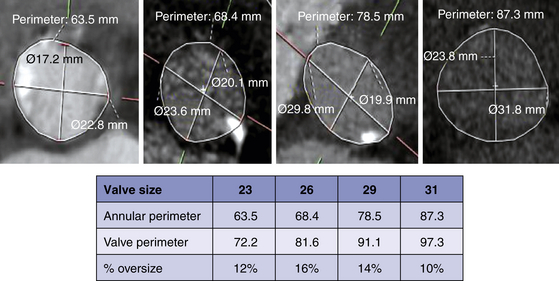
A, The annulus is used to measure the maximum annular diameter, perpendicular to the maximum diameter, and the annular area and annulus. The maximum length is 28.8 mm and the length perpendicular to the maximum length is 19.6 mm. The area was 473.2 mm2, and the perimeter was 82.3 mm. B, The maximum sinus of Valsalva diameters are comprised of the maximum diameter of the left coronary sinus (32.4 mm), right coronary sinus (29.5 mm), and noncoronary sinus (32.8 mm). The area of the sinus of Valsalva is 777.7 mm2, and the perimeter is 103.6 mm.
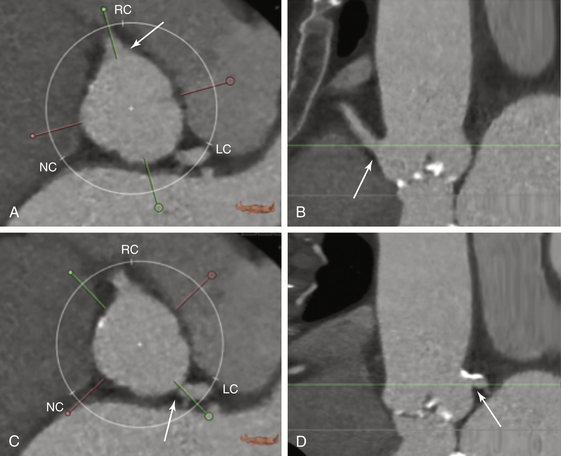
A, The height of the right coronary artery is identified (arrow). B, The height of the right coronary artery (arrow) from the annular base is 18.1 mm. C, The location of the left coronary artery is identified (arrow). D, The height of the left coronary artery (arrow) from the basal annulus is 15.2 mm.
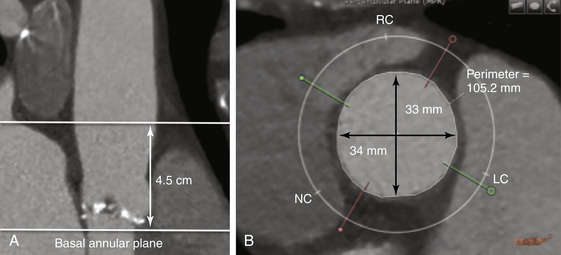
The aortic diameter is measured 4.5 cm above the basal annular plane (A), and the diameters are measured (B).
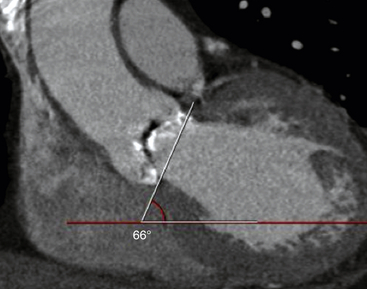
The angle of the horizontal plane and the aortic annulus is measured at 66 degrees.
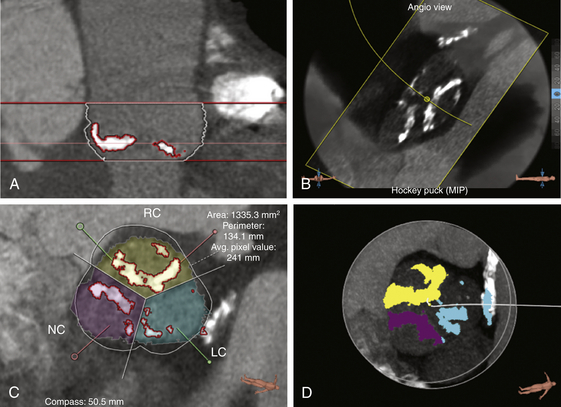
The stretch view shows dense calcification of the right coronary cusp, noncoronary cusp, and left coronary cusp. The stretch view (A), angiographic overlay (B), cross-sectional view (C), and “hockey puck” view (D) allow quantification of the calcium in the aortic valve.
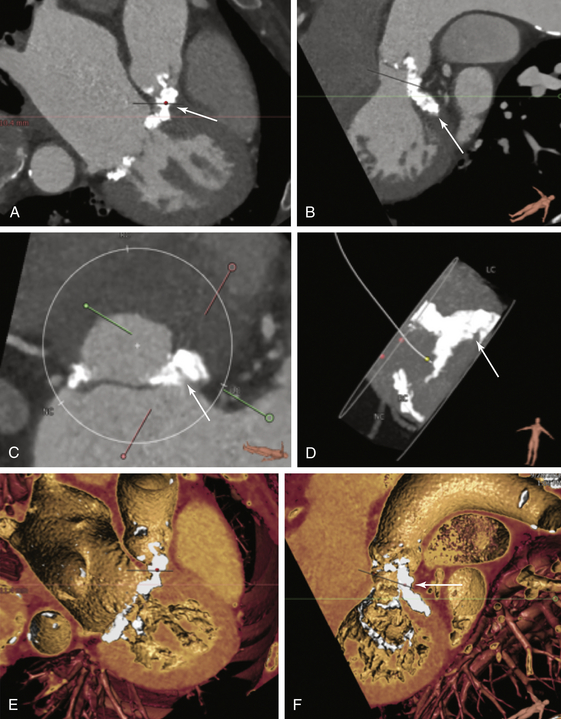
Dense calcification of the left coronary annulus is seen (A-D, arrows). Volume rendering of the mitral valve shows dense calcification extending from the left coronary sinus to the mitral annulus (E and F). This extent of calcification would be suboptimal for CoreValve transcatheter heart valve placement.
Vascular Criteria
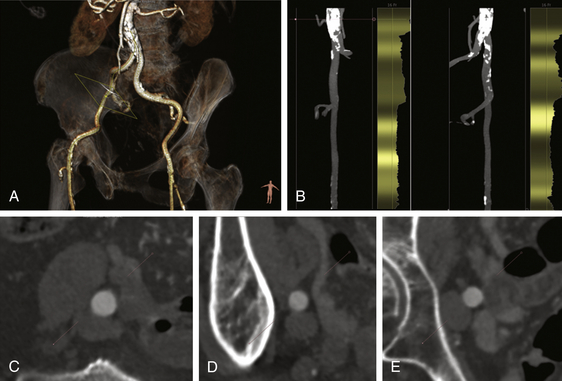
A, A reconstructed view of iliofemoral anatomy with slight obliquity shows the vessel tortuosity. B, The stretched views with overlay of calcification and vessel diameter allow determination of the minimal lumen diameters. C, Based on centerline reconstructions, the common iliac, external iliac (D), and proximal common femoral arterial (E) measurements are obtained.
Thoracic Key
Fastest Thoracic Insight Engine