Chapter 48 Sarcoidosis
Genetic Factors
Racial differences in incidence rates and disease clustering in families support the hypothesis that heredity contributes to sarcoidosis etiology. Having a first-degree relative with sarcoidosis increases the risk for the disease approximately five-fold. Screening for sarcoidosis among relatives is not recommended, because less than 1% will be found to have the disease. Several human leukocyte antigen (HLA) associations with sarcoidosis have been reported (Table 48-1), but HLA seems more likely to influence phenotype than susceptibility. The major histocompatibility complex (MHC) class I allele HLA-B8 is associated with acute sarcoidosis. HLA-DQB1*0201 and HLA-DRB1*0301 are strongly associated with acute disease and good prognosis.
Table 48-1 Summary of Human Leukocyte Antigen (HLA) Association Studies of Sarcoidosis
| HLA | Risk Alleles | Finding |
|---|---|---|
| HLA-A | A*1 | Susceptibility |
| HLA-B | B*8 | Susceptibility in several populations |
| HLA-DPB1 | *0201 | Not associated with sarcoidosis |
| HLA-DQB1 | *0201 | Protection, Löfgren syndrome, mild disease in several populations |
| *0602 | Susceptibility/disease progression in several groups | |
| HLA-DRB1 | *0301 | Acute onset/good prognosis in several groups |
| *04 | Protection in several populations | |
| *1101 | Susceptibility in whites and African Americans | |
| HLA-DRB3 | *1501 | Associated with Löfgren syndrome |
| *0101 | Susceptibility/disease progression in whites |
Investigators have studied non-HLA candidate genes that influence antigen processing, antigen presentation, macrophage and T cell activation, and cell recruitment. Table 48-2 lists non-HLA candidate genes studied to date. Although these candidates are logical on the basis of function, their associations with sarcoidosis have not been consistently reproduced.
Table 48-2 Non-HLA Candidate Genes Evaluated in Sarcoidosis
| Candidate Gene | Findings |
|---|---|
| Angiotensin-converting enzyme gene (ACE) | Increased risk for ID and DD genotypes |
| Moderate association between II genotype and radiographic progression | |
| CC chemokine receptor 2 gene | Associated with protection/Löfgren syndrome |
| Chemokine receptor 5 gene | CCR5Δ32 allele more common in patients treated with corticosteroid; refuted with haplotype analysis and larger sample |
| CD80, CD86 genes | No association detected |
| Clara cell 10-kDa protein gene | An allele associated with sarcoidosis and with progressive disease at 3-year follow-up |
| Complement receptor-1 gene | Association with the GG genotype for the Pro1827Arg (C[5507]G) polymorphism |
| Cystic fibrosis transmembrane regulator gene (CFTR) | R75Q increases risk |
| Cytotoxic T lymphocyte antigen 4 (CTLA4) | No association with sarcoidosis |
| Heat shock protein 70–like gene | HSP(+2437)CC associated with susceptibility/Löfgren syndrome |
| Inhibitor kappa B-alpha gene | Associated with −297T allele |
| Allele −827T in stage II | |
| IL1α gene | The IL-1α -889 1.1 genotype increased risk |
| IL-4 receptor gene | No association detected |
| IL-18 gene | Genotype −607CA increased risk over AA |
| Interferon-γ gene | IFNA17 polymorphism (551T→G) and IFNA10 [60A]− IFNA17 [551G] haplotype increase risk |
| Macrophage migration inhibitory factor gene | No association with 5-CATT in Irish population |
| Natural resistance–associated macrophage protein gene (NRAMP) | Protective effect of (CA)(n) repeat in the immediate 5′ region of the NRAMP1 gene |
| Toll-like receptor-4 gene (TLR4) | Asp299Gly and Thre399Ile mutations associated with chronic disease |
| Transforming growth factor gene (TGF) | TGF-β2 59941 allele, TGFβ3 4875 A and 17369 C alleles were associated with fibrosis on chest radiograph |
| Tumor necrosis factor-α gene | Genotype −307A allele associated with erythema nodosum/Löfgren syndrome and −857T allele with sarcoidosis |
| −307A not associated in African Americans | |
| Vascular endothelial growth factor gene | +813 CT and TT genotypes associated with protection |
| Vitamin D receptor gene (VDR) | BsmI allele associated with sarcoidosis |
Pathophysiology
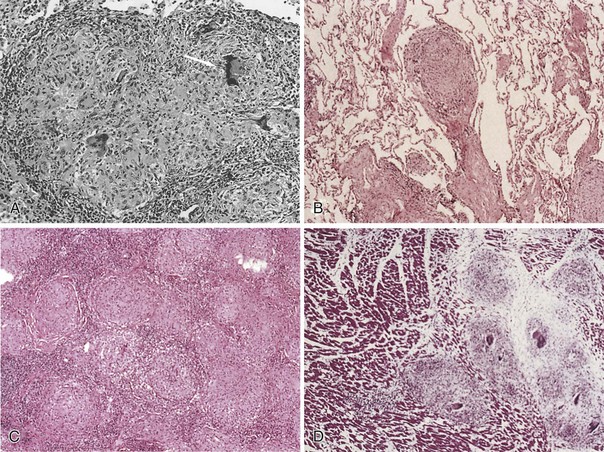
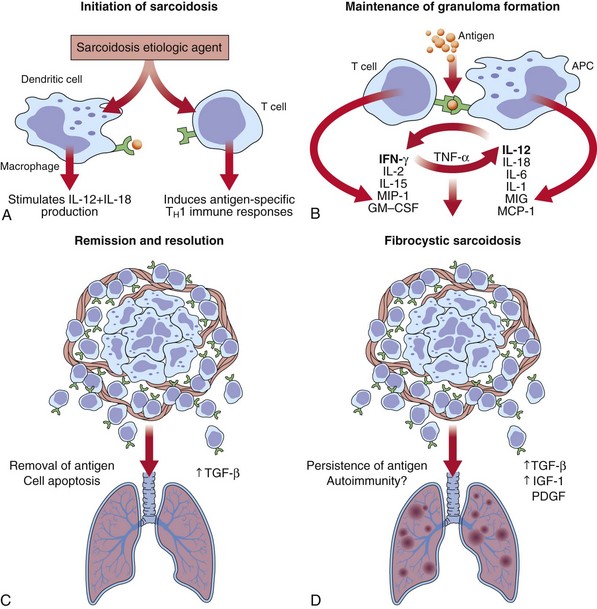
Sarcoidosis is a multisystem inflammatory disease characterized by T lymphocyte infiltration, granuloma formation, and microarchitecture distortion. The events leading to granuloma formation probably begin with antigen presented to T lymphocytes by way of MHC class II peptide in a genetically predisposed host (Figure 48-1). Serum amyloid A may have a role in the immune response in sarcoidosis and is capable of eliciting immune responses and triggering cytokine release through interaction with Toll-like receptors (TLRs). The oligoclonal T cell repertoire in sarcoidosis (α/β and δ/γ receptors) suggests that triggering antigens favor progressive accumulation and activation of selective T cell clones. Initially, macrophages process the antigens, which are then presented to CD4+ T cells by class II MHC molecules. T cell activation occurs by means of T cell receptor (TCR), TLR, or cytokine or chemokine receptors. This leads to signal transduction and activation of transcription factors that promote gene expression controlling T cell proliferation, differentiation, and apoptosis and produce proinflammatory cytokines and chemokines. T cell signal transduction pathways are potential therapeutic targets in sarcoidosis. Macrophages, in the face of chronic cytokine stimulation, differentiate into epithelioid cells, gain secretory and bactericidal capability, lose some phagocytic capacity, and fuse to form multinucleate giant cells. In more mature granulomas, fibroblasts and collagen encase the ball-like cell cluster. As granulomas accumulate, alterations in organ architecture and function occur.
Granulomas can resolve with little clinical consequence or may progress to fibrosis. Acute granulomatous and chronic fibrotic sarcoidosis probably represent different immunopathogenic processes, which remain to be defined (Figure 48-2). A switch from TH1 to TH2 cytokine profile, along with other mediators such as interleukin-6 (IL-6), transforming growth factor-β (TGF-β), osteopontin, and insulin growth factors (IGFs), is suggested to result in fibrosis. Sarcoidosis may develop or worsen in patients with immune reconstitution during treatment of HIV infection or in those undergoing treatment with interferon.
Clinical Features
Pulmonary Manifestations
Chest Radiology
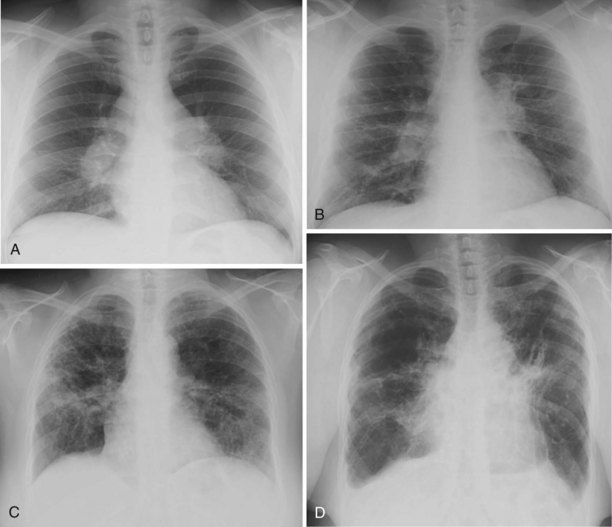
Chest radiographs obtained in patients with sarcoidosis are classified by appearance into four patterns (Figure 48-3). Unfortunately, these radiographic patterns have been termed “stages,” leading to the erroneous assumption that they represent disease stages rather than chest x-ray patterns.
Stage 0: Absence of radiographic abnormalities
Stage I: Bilateral hilar and/or mediastinal adenopathy without pulmonary parenchymal abnormalities
Stage II: Hilar and/or mediastinal lymphadenopathy with pulmonary parenchymal abnormalities (generally a diffuse interstitial pattern)
Stage III: Diffuse parenchymal disease without nodal enlargement
Stage IV: Pulmonary fibrosis with evidence of volume loss or cystic or honeycomb changes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree