Fig. 36.1
The Edwards SAPIEN transcatheter heart valve. The valve (shown en face on the left and from the side on the right) is available in 23 or 26 mm diameter. It consists of three bovine pericardial cusps mounted into a stainless steel balloon expandable stent
The delivery system used with the SAPIEN valve is the Retroflex III. It is a tapered nose cone-shaped balloon catheter with a deflectable tip (Fig. 36.2). It requires either a 22 or 24 Fr hydrophilic sheath for the 23 and 26 mm valves, respectively. The hub of the guiding catheter has a control knob, which can deflect the catheter through passage into the right ventricular outflow tract (RVOT) (Fig. 36.2).
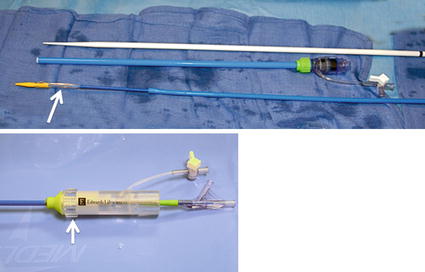

Fig. 36.2
The delivery system that includes the 35 cm-long delivery sheath and dilator and the RetroFlex delivery catheter that has a tapered steerable tip (shown in the top panel) which facilitates valve crossing; white arrow indicates the location of the valve on the catheter. The handle of the Retroflex catheter (shown in the bottom panel) has a knob (arrow) to steer the tip of the catheter
Currently, the next generation of the SAPIEN valves, the SAPIEN XT, is being evaluated for use in the aortic position in the USA with the PARTNER II trial. In this newer valve, the stent material has been changed from stainless steel to a cobalt chromium alloy, which allows for a smaller delivery profile and sheath. In addition, the SAPIEN XT valve is available in a 3rd size, a 29 mm diameter. The valve is available for use outside the USA, and there are case reports of this valve being used in the pulmonic position [1]; however, there is no available long-term data for this newer generation valve.
36.2 Anatomic Description and Pathophysiology
Congenital heart disease affects up to 1 in every 100 live births in the USA, and the tPVR procedure has provided a less invasive option to many of these patients instead of an additional cardiac surgery. It is performed primarily in patients with an RV to PA conduit and/or a bioprosthetic pulmonic valve.
In patients with a congenital RVOT obstruction, surgical implantation of an RV to PA conduit has allowed for the treatment and palliation of complex congenital heart disease that was previously untreatable. It has thereby contributed to the current survival rate of over 85 % of congenital heart disease patients into adulthood. Patients with cardiac anomalies afflicting the RVOT including pulmonary atresia with ventricular septal defect, tetralogy of Fallot, and truncus arteriosus need surgical correction with a conduit in the early neonatal period to improve blood flow to the lungs. Conduits are also used in patients with congenital aortic valve abnormalities, when undergoing the Ross procedure (autotransplantation of the native pulmonic valve in the aortic position and placement of a conduit between the right ventricle and pulmonary artery instead of the pulmonic valve used for the aortic position).
TPVR may also be performed in patients whose native RVOT was repaired surgically with a patch, such as patients with less severe forms of tetralogy of Fallot. The native RVOT can be stented, thereby creating a “conduit” between the RV and PA prior to valve implantation. Quite honestly, it is this group of patients we believe that constitutes the largest population who may benefit from such technology.
36.3 Clinical Scenarios
Conduit degeneration and prosthetic valve dysfunction are indolent disease processes. Symptoms of RVOT obstruction may develop slowly over the course of several years, if at all, before intervention is indicated. Symptoms typically include shortness of breath, fatigue, and symptoms of heart failure. Patients may also present with dizziness, syncope, or even sudden cardiac death if arrhythmias are present. However, many patients who are followed routinely by a cardiologist may develop conduit or prosthetic valve dysfunction and remain asymptomatic for many years. It is then up to the treating cardiologist to determine the optimal time for tPVR or surgery, based on the indications outlined in the next section.
36.4 Indications and Patient Selection
The 2010 American Heart Association statement on the Indications for Cardiac Catheterization and Intervention in Pediatric Cardiac Disease was expanded to include a class IIa indication for tPVR [1]. It recommends that “It is reasonable to consider percutaneous pulmonary valve replacement in a patient with an RV-to-PA conduit with associated moderate to severe pulmonary regurgitation or stenosis provided the patient meets inclusion/exclusion criteria for the available valve. (Level of Evidence: B).”
The inclusion and exclusion criteria for the SAPIEN valve trial are summarized in Table 36.1. These criteria were based on surgical indications for RVOT revision. However, it is important to note that there is some controversy regarding the optimal timing of surgery to prevent irreversible RV damage. The typical criteria that we use for asymptomatic patients include a pulmonary regurgitant fraction of >40 %, RV ejection fraction <40 %, and an indexed RV end-diastolic volume >150 ml/m2 as determined by cardiac MRI. However, if the patient is symptomatic due to severe pulmonary regurgitation or stenosis, then such criteria is not strictly enforced. Furthermore, the QRS duration in patients with severe pulmonary regurgitation should be taken into account. A QRS duration >180 ms is associated with ventricular arrhythmias, and sudden death and is considered an indication for intervention.
Table 36.1
Inclusion and exclusion criteria for the Edwards SAPIEN valve trial
Inclusion criteria |
Weight >35 kg |
In situ conduit >16 and <24 mm |
Dysfunctional RVOT conduit: |
>3+ PR by transthoracic echocardiogram |
Pulmonary regurgitant fraction >40 % |
With or without pulmonic stenosis |
Exclusion criteriaa |
Active infection requiring antibiotics |
History of or active endocarditis |
Intravenous drug abuse |
Preexisting prosthetic heart valve in any position |
Pregnancy |
Severe chest wall deformity |
Echocardiographic evidence of intracardiac mass, thrombus, or vegetation |
Known intolerance to aspirin or heparin |
36.5 Treatment Options
In adults, conduit replacement becomes necessary on average 10–15 years postsurgical implantation, but in children this time interval may be considerably shorter. Therefore, patients who had their first conduit placed during infancy may require four or more operations over their life span. Given the significant morbidity and mortality involved in redo operations in the setting of RV failure, a less invasive alternative is desirable.
TPVR is therefore a good option for patients requiring pulmonic valve intervention who meet the criteria listed above. It is important to remember, however, that even when patients meet criteria for tPVR and are seeking out a less invasive alternative to surgery, if their anatomy is not suitable (i.e., conduit or RVOT is too large or too small for available valves or not a long enough landing zone between the MPA and branch PAs), surgical conduit or valve replacement is still the gold standard.
There are currently two available valve systems for tPVR in the USA. Other than the SAPIEN valve, which is the focus of this chapter, the Melody valve is widely used in the USA for tPVR. The Melody valve (previously described in this book) is made of a bovine internal jugular vein and valve, sewn inside a platinum-iridium stent.
Both available valve systems have their unique benefits and drawbacks as summarized in Table 36.2. The SAPIEN valve is available in larger sizes than the current Melody system and therefore may be appropriate for placement in larger conduits, which may be found in older patients. It is also important to remember that it is not the original conduit size, but the degree of narrowing which determines the final size of the valve implanted. The SAPIEN valve has a shorter height than the Melody valve, which may be beneficial in certain anatomies; however, pre-stenting is necessary in order to give an adequate landing zone. The Melody delivery system, however, is less bulky, and the retractable sheath protects the valve until it is deployed in the desired location. The bulkier delivery system of the SAPIEN valve makes it potentially more difficult to implant, especially in patients with a tortuous RVOT. Careful consideration must be given to the likelihood of procedural success before attempting valve implantation because the SAPIEN system does not use a covering sheath; therefore, once it exits its short delivery sheath (35 cm) positioned in the inferior vena cava, it may be difficult to retract inside the sheath.
Table 36.2
Comparison of Melody and the SAPIEN valves
Characteristic | Melody valve | SAPIEN valve |
|---|---|---|
Stent material | Iridium 10 %, platinum 90 % | Stainless steel |
Valve material | Bovine jugular vein | Bovine pericardium treated with ThermaFix |
Available size (diameter) | 18–22 mm | 23, 26 mm (SAPIEN XT available in 29 mm outside the USA) |
Stent height | 34 mm | 14.5, 16 mm < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|