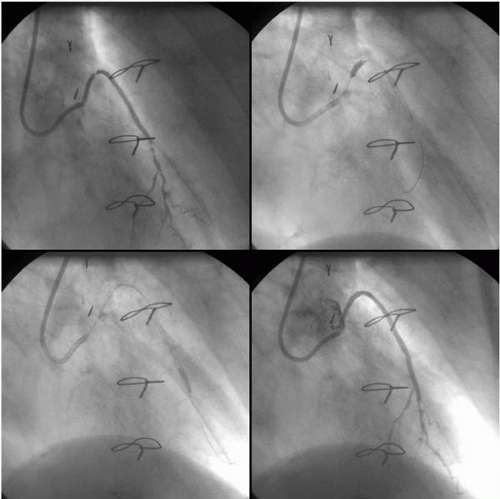
Saphenous Vein Graft Disease
John S. Douglas Jr.
Michael P. Savage
The successful application of surgical coronary revascularization in approximately .5 million patients annually for over three decades has resulted in millions of patients with diseased saphenous vein grafts (SVG). Percutaneous balloon angioplasty (BA) of ischemia-producing SVG lesions was recognized by Andreas Gruntzig, in his very early experience, to be associated with poor outcomes, leading him to say “Further experience will show whether we should eliminate this lesion from consideration” (1). To this day, SVG lesions remain one of the most challenging problems confronting interventional cardiologists. Procedures performed in patients with SVG lesions are often more complex and associated with higher risk than native coronary interventions and are significantly less likely to yield long-term benefit. These factors, higher risk and less benefit, cause decision making to be critically important in this difficult patient subgroup.
UNIQUE AND CHALLENGING FEATURES OF SAPHENOUS VEIN GRAFTS
The inferiority of SVGs compared to the atherosclerosisimmune internal mammary artery graft has long been recognized. However, surgeons continued to rely heavily on SVGs due to the lack of sufficient numbers of arterial conduits and the heightened technical challenges of multiple arterial anastomoses. Three different pathologic entities contribute variably to vein-graft narrowing and occlusion: intimal hyperplasia, thrombosis, and atherosclerosis. The pathology that the interventionalist encounters is heavily dependent on patient factors and the length of time since surgical implantation. Historically, stenosis and/or thrombotic occlusion of SVGs occurred in about 7% of patients during the first week, 15% to 20% during the first year, 1% to 2% per year during the next 6 years, and 4% per year from years 6 to 10 after surgery. By the tenth year, only 40% of patent grafts were free of significant stenoses (2, 3, 4). Stenoses appearing in SVGs within a few years of surgery are related to technical problems and/or intimal hyperplasia, but beginning 3 to 5 years postoperatively, atherosclerotic lesions begin to appear. The characteristics of these graft atheroma, which are frequently bulky and friable, significantly increase the risk of procedure-associated atheroembolic myocardial infarction (MI). This is true for percutaneous and surgical revascularization procedures. An inability to accurately predict the pathology that is present based on an assessment of angiographic features and the common presence of angiographically “silent” thrombus in varying states of organization has led to a recommendation for the routine use of embolic protection strategies whenever feasible during percutaneous treatment of de novo lesions in old SVGs. Unlike native coronary artery stent sites, which are protected from both atherosclerosis and intimal hyperplasia after the first year, percutaneously treated SVG sites may continue to progressively renarrow over time, and a marked tendency exists for progression of lesions at other sites within the same graft, thus resulting in high late cardiac event rates (5,6).
WEIGHING REVASCULARIZATION OPTIONS
A preference for percutaneous coronary intervention (PCI) on an SVG is often based on the higher risk and morbidity of reoperative coronary surgery (in-hospital mortality of about 7% for first reoperations) (7) and an incomplete
understanding of the procedural risk and poor durability of SVG interventions using balloons and bare metal stents. Whenever possible, consideration should be given to PCI of a native coronary artery rather than an old SVG, and this may involve recanalizing a chronic total occlusion of the native coronary. When the potential target for SVG PCI is an old SVG to the left anterior descending (LAD) coronary artery, reconsideration of surgery may be appropriate, especially when the internal mammary artery is available as a conduit, since the outcome of the LAD graft frequently determines the outcome of the patient (Fig. 38.1). The treatment of multiple SVG lesions, diffusely diseased SVGs, and/or occluded SVGs in patients with good surgical targets and acceptable surgical risk may be inappropriate given the poor long-term outcomes of PCI in these conditions. At Emory University and at the Cleveland Clinic, second surgical coronary revascularizations were required in about 3% of patients at 5 years and 12% by 12 to 15 years after surgery, with an average time to redo coronary artery bypass graft (CABG) of 11.5 years (8, 9, 10). These rates of reoperative surgery should be lowered by greater use of arterial grafts, contemporary lipid-lowering therapy, and antiplatelet agents. Among approximately 3,500 patients followed after a first CABG at Emory University, the 5-, 10-, and 12-year freedom from PCI was 0.98, 0.88, and 0.78 respectively (9). When outcomes of 2,613 post-CABG patients who underwent PCI were compared to 1,561 patients who were treated with reoperative surgery at Emory University, in-hospital outcomes were better with PCI for mortality (1.1% versus 6.9%, p <0.001), Q-wave infarctions (1.4% versus 5.4%, p <0.001), stroke (0% versus 2.8%, p = 0.27), and costs ($8,500 versus $24,200, p <0.01) (11,12). Correlates of long-term mortality after PCI included ejection fraction, heart failure, age, graft intervention, diabetes, and time from surgery. By 5 years, approximately half of PCI patients required either PCI or CABG, and survival was better in patients with native vessel interventions than in graft interventions (77% versus 68%, p <0.0001). A better outcome with native vessel PCI, however, was not confirmed in the Mayo Clinic experience after correction for baseline differences (13). An assessment of the long-term outcome of 2,256 patients who underwent PCI of SVG lesions at Emory University published recently highlighted the poor 5-year survival and event-free survival of diabetic patients compared to non-diabetic patients (0.60 versus 0.82, p = 0.001 and 0.23 versus 0.37, p = 0.001, respectively) (14). Whether
drug-eluting stents (DES) will have a major impact on outcomes following SVG PCI (discussed in an upcoming section) remains to be determined.
understanding of the procedural risk and poor durability of SVG interventions using balloons and bare metal stents. Whenever possible, consideration should be given to PCI of a native coronary artery rather than an old SVG, and this may involve recanalizing a chronic total occlusion of the native coronary. When the potential target for SVG PCI is an old SVG to the left anterior descending (LAD) coronary artery, reconsideration of surgery may be appropriate, especially when the internal mammary artery is available as a conduit, since the outcome of the LAD graft frequently determines the outcome of the patient (Fig. 38.1). The treatment of multiple SVG lesions, diffusely diseased SVGs, and/or occluded SVGs in patients with good surgical targets and acceptable surgical risk may be inappropriate given the poor long-term outcomes of PCI in these conditions. At Emory University and at the Cleveland Clinic, second surgical coronary revascularizations were required in about 3% of patients at 5 years and 12% by 12 to 15 years after surgery, with an average time to redo coronary artery bypass graft (CABG) of 11.5 years (8, 9, 10). These rates of reoperative surgery should be lowered by greater use of arterial grafts, contemporary lipid-lowering therapy, and antiplatelet agents. Among approximately 3,500 patients followed after a first CABG at Emory University, the 5-, 10-, and 12-year freedom from PCI was 0.98, 0.88, and 0.78 respectively (9). When outcomes of 2,613 post-CABG patients who underwent PCI were compared to 1,561 patients who were treated with reoperative surgery at Emory University, in-hospital outcomes were better with PCI for mortality (1.1% versus 6.9%, p <0.001), Q-wave infarctions (1.4% versus 5.4%, p <0.001), stroke (0% versus 2.8%, p = 0.27), and costs ($8,500 versus $24,200, p <0.01) (11,12). Correlates of long-term mortality after PCI included ejection fraction, heart failure, age, graft intervention, diabetes, and time from surgery. By 5 years, approximately half of PCI patients required either PCI or CABG, and survival was better in patients with native vessel interventions than in graft interventions (77% versus 68%, p <0.0001). A better outcome with native vessel PCI, however, was not confirmed in the Mayo Clinic experience after correction for baseline differences (13). An assessment of the long-term outcome of 2,256 patients who underwent PCI of SVG lesions at Emory University published recently highlighted the poor 5-year survival and event-free survival of diabetic patients compared to non-diabetic patients (0.60 versus 0.82, p = 0.001 and 0.23 versus 0.37, p = 0.001, respectively) (14). Whether
drug-eluting stents (DES) will have a major impact on outcomes following SVG PCI (discussed in an upcoming section) remains to be determined.
TECHNICAL APPROACHES TO SAPHENOUS VEIN GRAFT PERCUTANEOUS INTERVENTION
Balloon Angioplasty versus Stenting
Among Gruntzig’s first 50 patients, 5 had SVG lesions and 3 (60%) developed restenosis. Subsequently over 2,000 SVG BA procedures were reported from multiple centers, with acceptable mortality of 0.8%, Q-wave MI in 0% to 2.5%, and emergency surgery in 0.3% to 4% (15). Non-Q-wave infarction was reported in 13% of 599 patients who underwent 672 SVG graft dilatations at Emory University (16). Gruntzig’s early observations of a higher restenosis rate were confirmed; in the Emory experience, restenosis occurred in 64% of those dilated more than 5 years after surgery, in 61% treated at 1 to 5 years, and in 43% treated 6 months to 1 year after surgery. Restenosis occurred in 68% of proximal lesions, 61% of mid-vein graft lesions, and 45% of distal anastomotic lesions (p <0.06). The most favorable long-term outcomes occurred following BA of distal anastomotic lesions treated within a year of surgery, where restenosis was only 22%, and this strategy has remained the treatment of choice for this small subgroup of patients. Canos et al. reported that PCI of SVGs within 1 year of CABG compared to older SVGs (mean 8.8 years) had higher target-vessel revascularization (TVR) (38.6 versus 26.6, p = 0.04) (17). Ellis et al., in a careful follow-up of 103 patients after successful SVG intervention, reported event-free survival in 47% at 12 months and 25% at 36 months (6). In this study, 82% of events that occurred within the first year were attributed to the target SVG and two-thirds to the target lesion. Importantly, events after 1 year were more often attributed to untreated sites that had mild narrowing of 41% to 50% at the time of intervention. Although some have used this preliminary data to support the treatment of additional sites with borderline stenoses, the more important implications are the higher event rates when disease is nonfocal, the negative effects of selection of patients for percutaneous SVG intervention, and the need for subsequent surveillance strategies following SVG PCI. These observations also underscore the importance of secondary prevention measures, especially lipid lowering, to reduce the risk of disease progression at untreated sites.
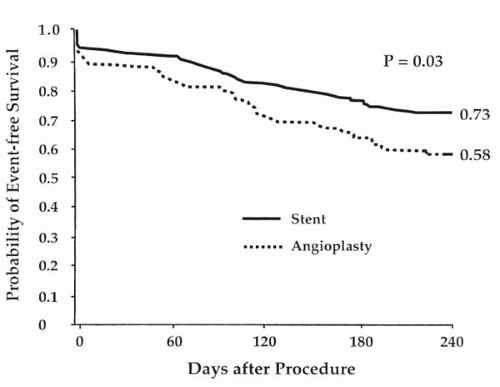
The use of a number of different bare metal stent (BMS) designs has been reported in SVG PCI. Safian compared the self-expanding Wallstent with the balloon-mounted Palmaz-Schatz stent in a randomized trial and reported similar outcomes (18). A multicenter registry of the use of the Palmaz-Schatz stent in SVG PCI reported outcomes of 589 patients, with 97% procedural success, stent thrombosis in 1.4%, in-hospital mortality in 1.7%, urgent CABG in 0.9%, and restenosis at 6 months in 18% for de novo lesions and 46% for restenotic lesions (19). Restenosis was more frequent in ostial SVG lesions than at other sites (61% versus 28%, p = 0.003). In the Saphenous Vein De Novo (SAVED) trial, 220 patients with new SVG lesions and angina or objective evidence of ischemia were randomly assigned to the implantation of Palmaz-Schatz stents or standard BA (20). Patients with lesion lengths greater than two stents, MI within 7 days, or evidence of intragraft thrombus were excluded. Patients assigned to stenting had a higher rate of procedural efficacy, defined as a reduction in stenosis to less than 50% of the vessel diameter with the assigned therapy (92% versus 69%, p <0.001). More bleeding occurred in the stent group due to warfarin anticoagulation. In-hospital major complications were otherwise similar in the two groups, although a trend was observed toward fewer non-Q-wave infarctions in the stent group (2% versus 7%). Whether stents significantly reduce atheroembolization is not certain, but this is a possible explanation for this trend and has led some operators to prefer a strategy of direct stenting in SVGs (21). In the SAVED trial, patients assigned to stents had a larger increase in lumen diameter immediately (1.92 versus 1.21 mm, p <0.001) and a greater net gain in lumen diameter at 6 months (0.85 versus 0.54 mm, p = 0.002). Restenosis occurred in 37% of stented patients and in 46% of the angioplasty group (p = 0.24). Event-free survival at 240 days (freedom from death, MI, or repeat revascularization) was significantly higher in the stent group (73% versus 58%, p <0.03) (Fig. 38.2). Late lumen loss was significantly greater in patients who had high-pressure (≥16 atm) stent expansion, indicating that routine high pressure may be undesirable in SVG PCI. Similarly, in an intravascular
ultrasound (IVUS) study of 226 SVG interventions, aggressive stent expansion to ≥100% of the reference lumen cross-sectional area was associated with significantly more non-Q-wave infarction (29 versus 17%, p = 0.05) and no improvement in TVR at 1 year (31% versus 26%, p = 0.3) (22). These studies suggest that less aggressive stent expansion is appropriate in SVGs.
ultrasound (IVUS) study of 226 SVG interventions, aggressive stent expansion to ≥100% of the reference lumen cross-sectional area was associated with significantly more non-Q-wave infarction (29 versus 17%, p = 0.05) and no improvement in TVR at 1 year (31% versus 26%, p = 0.3) (22). These studies suggest that less aggressive stent expansion is appropriate in SVGs.
The results of contemporary SVG PCI interventions were reported by Hong et al., who compared 1990-1994 patients with those treated in 1995-1998, noting similar initial results, but better 1-year event-free survival in the more recent cohort (71% versus 59%, p <0.001) and a protective effect of stent implantation (23). It was noted, however, that among 1,062 patients treated at William Beaumont Hospital from 1993 to 1997, 89 (8%) died in-hospital; and, at 3-year follow-up, another 92 patients (9%) had died and event-free survival was only 47% (24). At a median follow-up of 18 months at the Ottawa Heart Institute, only 44% were event-free (25) and, in an analysis of 627 SVG patients in five large randomized trials, adverse outcomes (mortality and MI) were twice that of native vessel PCI; the use of glycoprotein IIb/IIIa platelet receptor inhibitors did not reduce major adverse cardiac event (MACE) rates at 30 days or 6 months (26). These disappointing outcomes characterize the results of BMS in SVG PCI in the mid to late 1990s.
Based on the above trial data and on accrued clinical experience, operational techniques for SVG stenting have evolved in recent years. In contrast to native coronary stenting, where high-pressure deployment and oversized balloons are routinely used to achieve a maximal residual lumen diameter, optimal outcomes in SVG PCI dictate a modified approach. Important technical strategies include the routine use of direct stenting, avoidance of atheroablative devices, restrictive use of very high-pressure inflations (≥16 atm), and refraining from excessive balloon oversizing to avoid a deleterious “cheese grating” effect as well as graft perforation. By minimizing catheter manipulations and associated barotrauma, complications can be reduced and long-term outcome optimized.
Embolic Protection
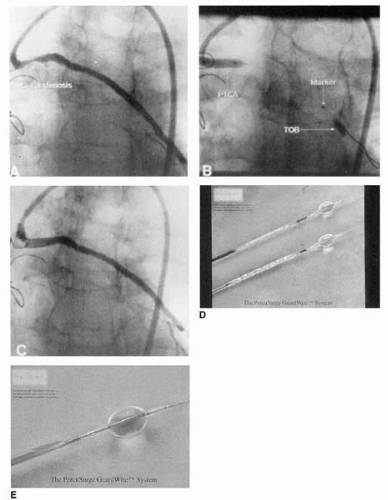
Saber et al. studied 32 patients who died within 3 weeks of PCI. They reported that 81% had histologic evidence of microembolization, compared with 2% of control patients (27). But only recently has there been increased awareness of the importance of embolization in atherosclerotic vascular disease and especially in SVG PCI, where periprocedural MI occurred in about 20% of straightforward, single-lesion, single-stent procedures (28, 29, 30, 31). The rate of MI and procedural risk was shown to increase with lesion complexity and length and estimated plaque volume (32), and when MI occurred, an increased 30-day mortality approaching 15% was observed (31). In approximately 1,000 patients who underwent SVG PCI, creatine kinase (CK-MB) elevation was the best independent predictor of late mortality (33). Potential approaches to reduce embolic MI in SVG PCI include strategies to remove thrombus prior to PCI, exclusion of debris with use of a covered stent (see Covered Stents), or capturing debris that has been liberated by use of filters or occlusion-aspiration techniques. The PercuSurge GuardWire system utilizes a hollow 0.014-inch PTCA wire incorporating a compliant, inflatable distal occlusion balloon (Fig 38.3). During occlusion of the distal graft, stent implantation is performed followed by aspiration of the graft using a special monorail catheter. Webb et al. reported that, in 24 SVGs aged 8.7 ± 5 years, which were stented using this protective system, CK levels exceeded three times normal in only one patient, and 95% of aspirates had typical atherosclerotic debris including necrotic core, foam cells, cholesterol clefts, and fibrin matrix (34). It was, however, the 801 patient randomized SAFER trial that convincingly demonstrated the value of distal protection during SVG stenting. Thirty-day MACE was reduced by 42% with use of the GuardWire (9.6% versus 16.5%, p <0.001), primarily due to lower rates of MI (8.6% versus 14.7%, p = 0.008) (35).
The PercuSurge system, frequently applicable even in the presence of severe stenosis due to the relatively low profile of the GuardWire system, captures small particles and soluble vasoactive agents (endothelin and serotonin) and coagulation components (tissue factor, plasminogen activator inhibitor, prothrombin fragment 1+2, and thrombin-antithrombin complex) that have been shown to be liberated during SVG PCI (36). The removal of these vasoactive substances may contribute to the improved immediate postprocedure coronary flow and myocardial perfusion observed with PercuSurge compared to filter-based distal protection (37). Analysis of the benefit of PercuSurge relative to lesion length in SAFER showed that even with lesions <10 mm length, a 77% reduction in 30-day MACE was experienced (2.2% versus 8.1%) (38) and in the presence of baseline thrombus, the use of the GuardWire was associated with reduced complications (OR 0.624, p = 0.02) (39). The disadvantages of the PercuSurge GuardWire include the need to completely occlude the target SVG during stent deployment and aspiration, a requirement for a relatively long “parking” segment distal to the lesion, inability to protect side-branches, and the complexity and two-operator requirement of the procedure.
The efficacy of filter-based distal protection in SVG stenting compared to use of the GuardWire was tested in the FIRE Trial, a randomized study of 650 patients at 65 clinical sites (Fig. 38.4) (40). Thirty-day MACE was similar: 9.9% using filter compared 11.6% using PercuSurge, as was MI (9% versus 10%) and death (0.9% versus 0.9%). Analysis of the first 48 patients compared with the next 261 demonstrated trends toward higher MI in the initial patients (19% versus 10%) due to failure to ensure good apposition of the filter to SVG wall, positioning the filter
too distal, use of the filter in Y grafts, and carrying out additional procedures after removal of the filter (i.e., unprotected PCI) (41). The advantages of the FilterWire include ease of use, maintenance of flow and avoidance of ischemia during stenting, and good visualization. The disadvantages include the need to cross the lesion with a relatively bulky filter, which may necessitate predilating the lesion, a maneuver shown to increase the rate of Q-wave MI (4.9% versus 0.5%, p = 0.04) (42). In addition, small particles of less than 100μ and soluble factors are not captured, and a long distal parking segment is required. An additional filter-based study known as the TRAP trial, testing the TRAP filter system compared with unprotected stenting in SVGs, was terminated after 360 of the 460 planned patients had been randomized. No difference was observed in 30-day MACE between the two groups, but a trend toward lower MI was observed in the TRAP group (43). In an unpublished industry-generated survey of SVG PCI in the United States during 2003, approximately 1,000 SVG procedures were analyzed: 62% were performed without distal protection, 27% using the FilterWire, 7% with distal occlusion and aspiration, whereas 4% involved some form of aspiration or thrombectomy (44). This low use of distal protection occurred despite the well-documented benefits, even in focal SVG lesions. The authors recommend routine use of distal protection in virtually all suitable de novo vein-graft lesions in SVGs that have been in place for 3 or more years.
too distal, use of the filter in Y grafts, and carrying out additional procedures after removal of the filter (i.e., unprotected PCI) (41). The advantages of the FilterWire include ease of use, maintenance of flow and avoidance of ischemia during stenting, and good visualization. The disadvantages include the need to cross the lesion with a relatively bulky filter, which may necessitate predilating the lesion, a maneuver shown to increase the rate of Q-wave MI (4.9% versus 0.5%, p = 0.04) (42). In addition, small particles of less than 100μ and soluble factors are not captured, and a long distal parking segment is required. An additional filter-based study known as the TRAP trial, testing the TRAP filter system compared with unprotected stenting in SVGs, was terminated after 360 of the 460 planned patients had been randomized. No difference was observed in 30-day MACE between the two groups, but a trend toward lower MI was observed in the TRAP group (43). In an unpublished industry-generated survey of SVG PCI in the United States during 2003, approximately 1,000 SVG procedures were analyzed: 62% were performed without distal protection, 27% using the FilterWire, 7% with distal occlusion and aspiration, whereas 4% involved some form of aspiration or thrombectomy (44). This low use of distal protection occurred despite the well-documented benefits, even in focal SVG lesions. The authors recommend routine use of distal protection in virtually all suitable de novo vein-graft lesions in SVGs that have been in place for 3 or more years.
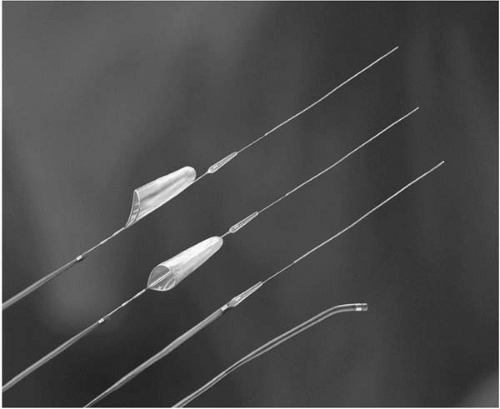 Figure 38.4. The FilterWire, shown to be not inferior to the PercuSurge GuardWire in the FIRE Trial. |
A third form of distal protection, known as Proxis, which utilizes a proximal occluding balloon, is currently undergoing clinical trials. The advantage of proximal occlusion includes the ability to protect during wire passage, protection of main vessel and side-branches, good visualization during stent deployment, and no need for the distal parking segment that can be a confounder with the use of FilterWire and PercuSurge.
Thrombectomy-Atherectomy
Debulking strategies utilizing atherectomy (45,46) and laser (47) have proved disappointing in SVGs due to increased complications and costs, without improvement in late outcomes. Mechanical thrombectomy techniques, however, can be very useful to avoid the thromboembolic MI, no-reflow phenomenon, and ischemic left ventricular dysfunction that frequently complicates PCI in the presence of thrombus (Fig. 38.5). Guide catheter thrombectomy (48,49), or the use of a specially designed aspiration catheter, such as the Export (AVE Medtronic, Santa Rosa, California), or PRONTO (Vascular Solutions, Minneapolis, Minnesota) are the simplest approaches. However, when a large amount of thrombus is not accessible to guide catheter aspiration, the AngioJet (POSSIS Medical, Inc., Minneapolis, Minnesota) is the most effective available device; it utilizes the Venturi effect to aspirate and fragment thrombus (50, 51, 52). In the randomized Vein Graft AngioJet Study (VeGAS 2), 350 patients received rheolytic thrombectomy or a urokinase infusion of 250,000 units for 15 to 30 minutes, followed by 20,000 units over 6 to 30 hours. Patients treated with AngioJet had fewer non-ST infarctions (12% versus 25%, p <0.05), less bleeding, reduced MACE (16% versus 33%, p <0.05) and lower cost by $3,500 compared to urokinase (50). Secondary effects induced by the AngioJet include bradycardia during right and circumflex coronary procedures related to adenosine release from red cells, ST-segment elevation due to potassium release from red cells, and hemoglobinuria if the device is utilized for a prolonged time. A novel dual-lumen thrombectomy catheter, known as the X-Sizer (ev3, Plymouth, Minnesota) has recently undergone a multicenter study. The X-Sizer is a helical cutter and aspiration device utilizing a 2,100 rpm motor drive; it has been shown to remove thrombus and soft atherosclerotic debris (53,54). In a recently published multicenter trial of 797 patients with diseased SVGs or thrombus-containing lesions, the use of the X-Sizer in SVGs was associated with fewer large MIs (5.4% versus 10.4%, p = 0.03), but a similar rate of all infarctions occurred (15.8% versus 16.6%, p = 0.77) (55). Some of the advantages of the X-Sizer are its ease of use, short setup time, and compatibility with usual PCI equipment. A multicenter trial
of ultrasound thrombolysis during SVG PCI was terminated prematurely due to higher 30 day MACE in the ultrasound arm (25% versus 12%) (56).
of ultrasound thrombolysis during SVG PCI was terminated prematurely due to higher 30 day MACE in the ultrasound arm (25% versus 12%) (56).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree