Contrast-enhanced ultrasound imaging is a radiation-free diagnostic tool that uses biocompatible ultrasound contrast agents (UCAs) to improve image clarity. UCAs, which do not contain dye, often salvage “technically difficult” ultrasound scans, increasing the accuracy and reliability of a front-line ultrasound diagnosis, reducing unnecessary downstream testing, lowering overall health care costs, changing therapy, and improving patient care. Two UCAs currently are approved and regulated by the US Food and Drug Administration. They have favorable safety profiles and risk/benefit ratios in adult and pediatric populations, including compromised patients with severe cardiovascular diseases. Nevertheless, these UCAs are contraindicated in patients with known or suspected right-to-left, bidirectional, or transient right-to-left cardiac shunts. These patients, who constitute 10% to 35% of the general population, typically receive no UCAs when they undergo echocardiography. If their echocardiographic images are suboptimal, they may receive inappropriate diagnosis and treatment, or they may be referred for additional diagnostic testing, including radiation-based procedures that increase their lifetime risk for cancer or procedures that use contrast agents containing dye, which may increase the risk for kidney damage. An exhaustive review of current peer-reviewed research demonstrated no scientific basis for the UCA contraindication in patients with known or suspected cardiac shunts. Initial safety concerns were based on limited rodent data and speculation related to macroaggregated albumin microspheres, a radioactive nuclear imaging agent with different physical and chemical properties and no relation to UCAs. Radioactive macroaggregated albumin is not contraindicated in adult or pediatric patients with cardiac shunts and is routinely used in these populations. In conclusion, the International Contrast Ultrasound Society Board recommends removal of the contraindication to further the public interest in safe, reliable, radiation-free diagnostic imaging options for patients with known or suspected cardiac shunts and to reduce their need for unnecessary downstream testing.
Ultrasound contrast agents (UCAs) are radiation-free substances that improve ultrasound image clarity. UCAs often are used to salvage “technically difficult” ultrasound scans, for example, when ultrasound signal transmission is impaired by obesity or other physical impediments. Contrast-enhanced ultrasound imaging and UCAs produce images in real-time using equipment that is portable and widely available. By increasing the reliability of front-line ultrasound scans, UCAs provide a more accurate initial diagnosis, reduce the need for downstream testing, lower overall health care costs, change therapies, and improve patient care without exposing patients to ionizing radiation or increasing the risk for nephrotoxicity. In the United States, 2 commercial UCAs, Optison (GE Healthcare, Milwaukee, Wisconsin) and Definity (Lantheus Medical Imaging, North Billerica, Massachusetts), are approved and regulated by the US Food and Drug Administration (FDA). The 2 UCAs currently are contraindicated in patients with known or suspected cardiac shunts, who constitute 10% to 35% of the general population. Physicians representing the International Contrast Ultrasound Society raised concerns about the current contraindication and its impact on patient care during a September 11, 2012, professional society briefing on the safety of UCAs to staff members of the Center for Drug Evaluation and Research at the FDA. International Contrast Ultrasound Society representatives committed to prepare a technical report to assess current peer-reviewed research. Key findings of this study include the following: (1) Current published research is replete with peer-reviewed studies demonstrating the safety and clinical benefits of commercial UCAs in adult and pediatric populations, including patients with severe cardiovascular diseases, (2) the contraindication is not evidence based, (3) initial safety concerns were based on limited rodent data that have not corresponded with human data along with speculation pertaining to a radioactively-tagged nuclear imaging contrast agent with physical and chemical properties unlike those of UCAs, (4) the nuclear imaging agent is not contraindicated in patients with known or suspected cardiac shunts, and (5) the cumulative weight of scientific evidence supports the use of UCAs in adult and pediatric patients with known or suspected cardiac shunts.
Noncommercial and Commercial Ultrasound Contrast Agents
All UCAs are suspensions of biocompatible gas-filled microbubbles or microspheres that reflect ultrasound signals. They are administered during a diagnostic ultrasound examination either intravenously or by direct catheter infusions (to the bladder, uterus, Fallopian tubes, etc.). UCAs may be used for parenteral injections (blood pool agents) or enteral injections (gastrointestinal or intraorgan injection, bladder or Fallopian tube infusions). UCAs traverse the microvasculature at physiologic transit times, generating contrast effects that highlight anatomic boundaries (vascular vs nonvascular tissues and organs) and define microvascular tissue perfusion. Contrast effects are produced by the interface between the gas and the surrounding shell, which creates an acoustic mismatch that increases the signal-to-noise ratio between the blood and tissue.
Presently, there are 2 types of UCAs: ncommercial hand-agitated UCAs (not regulated by the FDA), which are produced at the bedside by manually agitating a solution, such as saline, to create a suspension of ultrasound-reflective gas-filled microbubbles, and commercial UCAs (Optison and Definity), which are produced under controlled conditions regulated by the FDA. The commercial agents contain suspensions of microspheres composed of an outer shell (either albumin or a phospholipid) encapsulating a perfluorocarbon gas. Optison is composed of perflutren protein type A microspheres for injectable suspension. Definity is a perflutren lipid microsphere injectable suspension,
The first UCAs were hand-agitated suspensions of microbubbles created by trial and error. Claude Joyner is credited with describing the initial hand-agitated microbubbles, and Gramiak and Shah, in 1968, published the first report describing the use of these microbubbles as a UCA. Feigenbaum et al subsequently expanded this work and used manually agitated indocyanine green as a UCA.
Today, noncommercial UCAs are widely used in clinical practice, and their method of production has not changed since 1968. Typically, 2 syringes connected by a stopcock are used to rapidly mix a solution of bacteriostatic saline (9 cm 3 ) and room air (1 cm 3 ), producing a transient suspension of microbubbles measuring 31.6 ± 8.2 μm. Because the mean size of these manually agitated microbubbles may be 5 to 10 times larger than the mean size of the microspheres constituting commercial UCAs, they expose patients to larger volumes of gas and are limited in their ability to pass through lung capillaries, reducing visualization of the left-sided heart chambers. In addition, manually agitated microbubbles are variable in size and concentration, and because they do not contain stabilizing shells, they must be used within seconds to minutes.
The first generation of commercial, FDA-approved UCAs consisted of nitrogen-based gases encapsulated with protein shells (Albunex; Molecular Biosystems, Inc., San Diego, California) or finely milled particulate matter that was hydrophilic (Levovist; Bayer AG, Leverkusen, Germany). Coincident with the development of commercial UCAs, ultrasound equipment manufacturers developed newer, more sophisticated harmonic imaging software that produced significant enhancement of the signal-to-noise ratio while using a lower mechanical index.
Second-generation commercial UCAs (Optison and Definity) use perflutren gas, a low-soluble gas with high molecular weight, to extend in vivo persistence. The perflutren microspheres are smaller and more stable than first-generation commercial UCAs and are capable of crossing the pulmonary capillary bed. This permits more efficient noninvasive imaging of the left-sided cardiac chambers and assessment of myocardial perfusion.
Third-generation “designer” UCAs are in development for molecular imaging. They are uniquely and specifically labeled to permit quantitative, physiologic localization (“molecular imaging”) of inflammation and related disease states.
In addition, fourth-generation therapeutic UCAs are in development and have achieved success in preclinical studies. Therapeutic UCAs will carry genes and drugs to tumors and specific organ systems throughout the body, serving as ultrasound-directed, site-specific drug or gene therapeutic delivery platforms. A summary of developmental or approved commercial UCAs is listed in Table 1 .
| Manufacturer | Name | Type | Development Stage |
|---|---|---|---|
| Accusphere | Imagify | Polymer/perfluorocarbon | Clinical development |
| Alliance/Schering | Imavist | Encapsulated perfluorocarbon | Clinical development |
| Bracco | SonoVue | Lipid/sulfur hexafluoride | Approved outside the United States |
| Bracco | SonoRx | Simethicone-coated cellulose | Approved outside the United States |
| Byk-Gulden | BY963 | Lipid/air | Clinical development |
| Lantheus Medical Imaging | Definity | Perflutren lipid microspheres | Approved for use |
| GE Healthcare | Optison | Perflutren protein type A microspheres | Approved for use |
| GE Healthcare/Daiichi | Sonazoid | Lipid/perfluorocarbon microspheres | Approved outside the United States |
| MBI/Mallinckrodt | Albunex | Sonicated albumin microspheres | Approved for use |
| Schering | Echovist | Reconstituted galactose granules | Approved outside the United States |
| Schering | Levovist | Galactose and palmitic acid granules | Approved outside the United States |
| Schering | Sonovist | Polymer/air | Clinical development |
Clinical Ultrasound Contrast Agent Use
Agitated saline solutions are widely used in adult and pediatric populations without regulatory oversight or contraindication. Echocardiography laboratories throughout the United States use agitated saline solutions during transthoracic echocardiography and transesophageal echocardiography to detect cardiac shunts, including patent foramen ovale, a condition occurring in approximately 10% to 35% of the general population. Radiology and neurology ultrasound laboratories also use noncommercial UCAs during transcranial Doppler imaging for the detection of cardiac shunts ( Figure 1 ). In addition, agitated saline solutions are used to evaluate atrial septal defects, sinus venosus defects, persistent left superior vena cava, intrapulmonary shunting, and exercise-induced transpulmonary shunting. Pediatric congenital cardiac defects also are routinely identified with noncommercial hand-agitated saline UCAs.
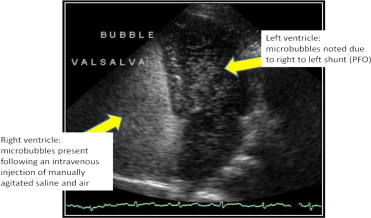
Commercial UCAs typically are used in the United States to enhance suboptimal echocardiographic studies for the delineation of left ventricular endocardial borders and opacification. An estimated 10% to 30% of all transthoracic echocardiographic studies are considered suboptimal. UCA enhancement of suboptimal echocardiographic exams is addressed by contrast-enhanced ultrasound echocardiographic guidelines adopted by the American Society of Echocardiography (in 2000 and 2008) and the European Society of Echocardiography (in 2009).
Commercial UCAs also are used off label during stress echocardiography and transesophageal echocardiography and for imaging the liver, prostate, brain, and other noncardiac structures. UCAs must be used off label for the enhancement of suboptimal stress echocardiographic studies unless alternative imaging plans are in place, on the basis of accreditation standards for adult echocardiography laboratories adopted in 2010 by the Intersocietal Accreditation Commission (known at the time as the Intersocietal Commission for the Accreditation of Echocardiography Laboratories). In addition to these intravenously administered UCA applications, nonintravenous off-label indications include use in hysterosalpingo-sonographic detection of Fallopian tube patency as well as voiding urosonography for the diagnosis of vesicoureteric reflux in pediatric patients. Outside the United States, where UCAs have a broader range of regulatory approvals, commercial UCAs also are used for the detection and assessment of myocardial perfusion, tumors, gastrointestinal disorders, and numerous other indications.
Clinical Ultrasound Contrast Agent Use
Agitated saline solutions are widely used in adult and pediatric populations without regulatory oversight or contraindication. Echocardiography laboratories throughout the United States use agitated saline solutions during transthoracic echocardiography and transesophageal echocardiography to detect cardiac shunts, including patent foramen ovale, a condition occurring in approximately 10% to 35% of the general population. Radiology and neurology ultrasound laboratories also use noncommercial UCAs during transcranial Doppler imaging for the detection of cardiac shunts ( Figure 1 ). In addition, agitated saline solutions are used to evaluate atrial septal defects, sinus venosus defects, persistent left superior vena cava, intrapulmonary shunting, and exercise-induced transpulmonary shunting. Pediatric congenital cardiac defects also are routinely identified with noncommercial hand-agitated saline UCAs.
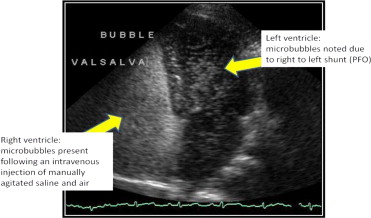
Commercial UCAs typically are used in the United States to enhance suboptimal echocardiographic studies for the delineation of left ventricular endocardial borders and opacification. An estimated 10% to 30% of all transthoracic echocardiographic studies are considered suboptimal. UCA enhancement of suboptimal echocardiographic exams is addressed by contrast-enhanced ultrasound echocardiographic guidelines adopted by the American Society of Echocardiography (in 2000 and 2008) and the European Society of Echocardiography (in 2009).
Commercial UCAs also are used off label during stress echocardiography and transesophageal echocardiography and for imaging the liver, prostate, brain, and other noncardiac structures. UCAs must be used off label for the enhancement of suboptimal stress echocardiographic studies unless alternative imaging plans are in place, on the basis of accreditation standards for adult echocardiography laboratories adopted in 2010 by the Intersocietal Accreditation Commission (known at the time as the Intersocietal Commission for the Accreditation of Echocardiography Laboratories). In addition to these intravenously administered UCA applications, nonintravenous off-label indications include use in hysterosalpingo-sonographic detection of Fallopian tube patency as well as voiding urosonography for the diagnosis of vesicoureteric reflux in pediatric patients. Outside the United States, where UCAs have a broader range of regulatory approvals, commercial UCAs also are used for the detection and assessment of myocardial perfusion, tumors, gastrointestinal disorders, and numerous other indications.
Ultrasound Contrast Agent Safety
The safety of Optison and Definity, the 2 FDA-approved UCAs, is well established. FDA advisory panels evaluated their safety most recently on June 24, 2008, and May 2, 2011, and the agency has 3 times downgraded UCA package insert contraindications and warnings. In addition, the peer-reviewed research is now replete with studies demonstrating the safety of commercial UCAs in pediatric and adult populations, including patients with congestive heart failure, pulmonary hypertension, and other severe cardiovascular diseases. Data also demonstrate the safety of UCAs in the pediatric population, with a low risk-to-benefit ratio and improved care in these young patients.
As noted, hand-agitated UCAs are produced at the bedside without the benefit of regulatory oversight. They contain microbubbles that are larger, more variable in size and less stable than commercially produced UCA microspheres. Nevertheless, hand-agitated UCAs have a low record of complications and generally are administered without hemodynamic or neurologic risk. Only isolated cases of transient adverse neurologic events have been reported, despite the continued high volume of hand-agitated microbubble procedures.
Safety data for hand-agitated UCAs include animal studies and clinical case reports. Animal data suggest that after mixing 9 cm 3 of agitated saline with 1 cm 3 of air, a large bolus of gas (20 ml/min) or a continuous bolus of gas (11 ml/min) injected intravenously would be required to generate intra-arterial bubbles. Other animal data demonstrate that injections of 2 ml of air in the radial artery are sufficient for passage into the cerebral system. In addition, case reports and case series have described neurologic events associated with the administration of an agitated air-saline mixture in patients with cardiac shunts. A further study of transcranial Doppler and echocardiographic evaluations demonstrated stroke symptoms in a series of 5 patients after the administration of manually agitated microbubble solutions; these cases represented a small sample of >3,000 manually agitated saline studies performed at the reporting institutions. A report of the American Society of Echocardiography assessed the risk for side effects from contrast (including saline and indocyanine green) at 0.062% and concluded that the low risk for adverse events is outweighed by the significant diagnostic benefits of contrast imaging.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


