Safety of Cardiac Resynchronization Therapy
Mikhael F. El-Chami
Angel R. Leon
Cardiac resynchronization therapy (CRT) improves symptoms and reduces mortality in patients with symptomatic congestive heart failure (CHF) and left bundle branch block (LBBB).1,2,3,4,5 The success of this technology as an effective adjunctive therapy in patients with CHF has led to the widespread adaptation of CRT. Hence, familiarity with the potential complications associated with this procedure will help ensure a safer implantation with lower risks to patients. This chapter will address safety of CRT by discussing the success and complication rates associated with this procedure.
IMPLANTATION SUCCESS RATE
Success of CRT implant ranges from 89% to 95.6%.6,7 Leon et al.7 reported on the outcome of CRT in over 2,000 patients enrolled in the MIRACLE study program [three completed randomized trials: the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) study, the MIRACLE Implantable Cardioverter-Defibrillator (ICD) study, and the InSync III study]. In these trials, CRT implant succeeded in 1,903 (91.6%) of 2,078 patients, with 35 (1.8%) requiring more than one attempt to achieve success. In a more recent report addressing the same issue,6 the procedure was successful in 390 out of 404 patients (95.6%) enrolled in the CAREHF study. CRT implant was successful in 349 patients (89.5%) at the first attempt, in 36 patients (9.2%) at the second attempt, and in 5 patients (1.3%) at the third attempt.
In the MIRACLE study program, the most common reasons for unsuccessful procedures were as follows: inability to access the coronary sinus (CS) in 69 patients (39.4%), acute dislodgement or unstable lead position in 59 patients (33.7%), and inability to obtain distal lead location in 52 (29.7%). Similarly, in the CARE-HF trial, inability to access the CS (42.8%) was the most common cause of procedural failure followed by inability to achieve stable lead position (32.6%), inability to access target veins (12.2.%), unacceptable stimulation threshold (8.2%), and phrenic nerve stimulation (4.1%).
In the MIRACLE study, operator experience predicted successful CRT implantation. The success rate for the first five implants at each center was 89.5% increasing to 94.6% for the next five implants, and continuing at 93.4% after the 10th implant. Furthermore, implant duration decreased with more experience. The mean implant duration decreased from 2.7 hours in the MIRACLE study to 2.3 hours in the InSync study (p <0.0001). (InSync study was conducted after the MIRACLE study.) In addition, the mean time to CS cannulation decreased from 11 minutes in the MIRACLE study to 6.5 minutes in the InSync study; the median fluoroscopy time decreased from 33 minutes in the MIRACLE study to 27.6 minutes in the InSync study.
The CARE-HF trial also looked closely at predictors of implantation success rate. The only predictor of success rate was implant center experience set empirically at </ = 10 implants per year versus > 10 implants per year. The first time success rate was 90% for experienced centers versus 82% for nonexperienced centers. The implantation success rate is reported to be higher in high-volume centers after the introduction of over the wire (OTW) technology, reached up to 98% in a single center experience (400 patients)8 and 97% in a recently published multicenter study, Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE trial).9
A lateral left ventricular lead position was achieved in 88% of patients (76% lateral or posterolateral and 12% anterolateral) but was anterior (great cardiac vein) in the remainder of patients enrolled in the CARE-HF study. The LV lead threshold was higher than the right ventricular (RV) and right atrial leads but remained relatively stable over time (Mean 1.23 in the MIRACLE study program and 1.25 in the CAREHF trail at implant). Similarly the LV lead sensing and impedance remained stable over time.
Overall, the success rate of CRT implantation is high, improves with experience, and is likely to improve with availability of newer delivery system and newer lead technology.
IMPLANT COMPLICATIONS
Infection, pocket complications, pneumothorax, or hemothorax are potential complications of any device implant
(Fig. 8.1). These complications do not appear to be more common with CRT than in conventional pacing or defibrillator system implant.7 For instance, in the mode selection trial in sinus node dysfunction10 and in the Canadian Trial of Physiologic Pacing (CTOPP)11 the incidence of pneumothorax was around 1.5%. Infection is reported in around 2% to 8% of device implant.12,13
(Fig. 8.1). These complications do not appear to be more common with CRT than in conventional pacing or defibrillator system implant.7 For instance, in the mode selection trial in sinus node dysfunction10 and in the Canadian Trial of Physiologic Pacing (CTOPP)11 the incidence of pneumothorax was around 1.5%. Infection is reported in around 2% to 8% of device implant.12,13
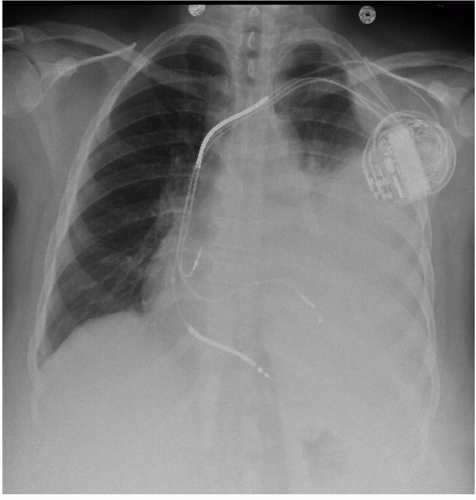 FIG. 8.1. A CXR revealing a patient with a hemothorax after CRT-D implant. Note the left pleural effusion obscuring the left heart border. |
In the CARE-HF study, a pneumothorax occurred in 1.3% of cases, while pocket infection complicated 0.6% of all CRT implant. A similar low rate of pneumothorax (<1%) and infection (1%) was reported in the MIRACLE study program.
Complications that are specific to CRT implant include:
LV lead dislodgement
Diaphragmatic stimulation
CS dissection and perforation
LV Lead Dislodgement
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


