Pericardiocentesis is useful in the diagnosis and treatment of pericardial effusive disease. To date, a number of methods have been developed to reduce complications and increase the success rate of the procedure. The aim of the present study was to evaluate the efficacy and the safety of echocardiography-guided pericardiocentesis under continuous echocardiographic monitoring in the management of pericardial effusion. We prospectively performed 161 pericardiocentesis procedures in 141 patients admitted from 1993 to 2015 in 3 centers. This procedure was performed for tamponade or large pericardial effusion in 157 cases and for diagnosis in 4 cases. A percutaneous puncture was performed where the largest amount of fluid was detected. To perform a real-time echo-guided procedure, a multi-angle bracket was mounted on the echocardiographic probe to support the needle and enable its continuous visualization during the puncture. The procedure was successful in 160 of 161 cases (99%). Two major complications occurred (1.2%): 1 mediastinal hematoma that required surgical drainage in a patient on anticoagulant therapy and 1 pleuropericardial shunt requiring thoracentesis. Seven minor complications occurred (4.3%): 1 pleuropericardial shunt, 1 case of transient AV type III block, 3 vasovagal reactions (1 with syncope), and 2 cases of acute pulmonary edema managed with medical therapy. No punctures of any cardiac chamber occurred, and emergency surgical drainage was not required in any case. In conclusion, echocardiography-guided pericardiocentesis under continuous visualization is effective, safe, and easy to perform, even in hospitals with low volumes of procedures with or without cardiac surgery.
Pericardiocentesis is a valuable technique in the diagnosis and treatment of patients with moderate or large symptomatic pericardial effusions and/or cardiac tamponade. To date, various methods have been used to cope with this problem, monitor the procedure, and reduce complication rates. Initial blind approaches are no longer justified. Pericardiocentesis assisted by 2-dimensional echocardiography was developed and described by Callahan at the beginning of the 1980s. This technique has been demonstrated to be simple, safe, and effective, as documented by the Mayo Clinic case series with 1,127 patients. In our study, we assessed the safety, efficacy, and feasibility of echocardiography-guided pericardiocentesis under continuous visualization of needle insertion.
Methods
We performed 161 pericardiocentesis procedures in 141 consecutive patients who presented from 1993 to 2015 at 3 hospitals with different interventional caseloads: San Gerardo Hospital, Monza, from 1993 to 2005 (n = 91) (7.3 pericardiocentesis procedures/year), Manzoni Hospital, Lecco, from 2010 to 2015 (n = 51) (10 pericardiocentesis procedures/year), and San L. Mandic Hospital, Merate, from 2009 to 2015 (n = 19) (3 pericardiocentesis procedures/year). San Gerardo Hospital has had a cardiac surgery department since 1999 and Manzoni Hospital has had a cardiac surgery department since 2009; San L. Mandic Hospital does not have cardiac surgery capability.
The present study includes data from the first 53 patients who were enrolled from 1993 to 2000 at San Gerardo Hospital and published in 2001. Data were prospectively recorded in a prespecified database that included age, gender, hemodynamic status, blood and pericardial effusion examination, and technique of pericardiocentesis execution. All procedures were performed in an intensive cardiac care unit. The technique has been described in previous reports. Patients were monitored with continuous electrocardiography, pulse oximetry, and noninvasive blood pressure. When possible, invasive monitoring with a jugular central venous catheter and a radial artery catheter was performed. In cases of hemodynamic instability with hypotension, volume expansion with 500 ml of isotonic saline was administered before the procedure. No routine pharmacologic treatment, specifically, neither sedation nor antibiotic prophylaxis was administered. Packed red cell units were available before starting nonemergency procedures. A preliminary echo study was performed to select the needle insertion site, defined as the point at which the effusion was closest to the thoracic wall and had the maximum thickness. The procedure was performed under aseptic conditions and local anesthesia with 2% lidocaine.
To allow a real-time echo-monitored procedure, a multi-angle bracket with 3 different angles was mounted on the probe to support the needle (in recent years, biopsy starter kit for GE 3S, 3S-RS, M3S, and M4S transducers; CIVCO USA, Kalona, Iowa). The operator could choose 3 different angles between the needle and the probe for the needle placement, to allow a good visualization of the needle tip in the ultrasound beam while reaching the pericardium. A closer angle is indicated in subcostal approach, where the path to reach the pericardium is longer. In the apical approach, a wider angle allows a better visualization of the needle in the short space separating the probe from the pericardium ( Figure 1 ).
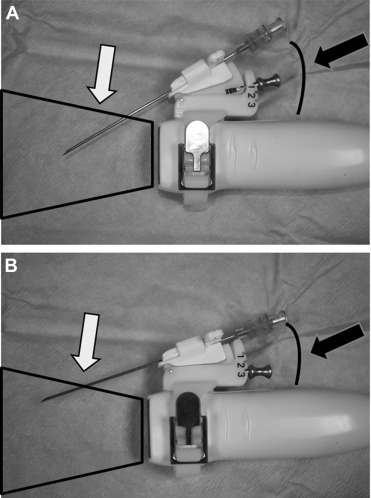
Once the desired angle was selected, a stainless steel pin locked the angle securely into position. Echocardiographic equipment has changed over time, but the technique that is now used has not been significantly modified. The echo probe was subsequently covered with a sterile sheath. An 18-gauge needle was inserted in the disposable support mounted on the covered probe (Ultra Pro II needle guide; CIVCO USA) to allow precise advancement on the plane of the ultrasound beam. In this way, we had a continuous visualization of the needle tip. Once the optimal position was found and the pericardiocentesis procedure was started, the echocardiography probe should not be moved anymore to avoid tearing the tissue. The needle was then connected to a syringe and was advanced by maintaining gentle aspiration.
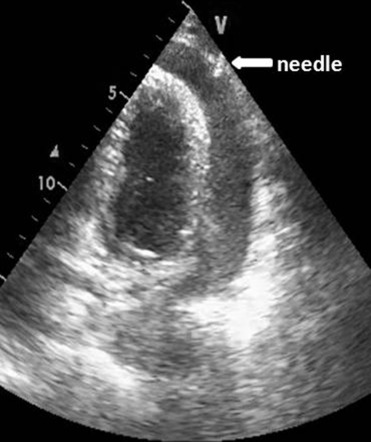
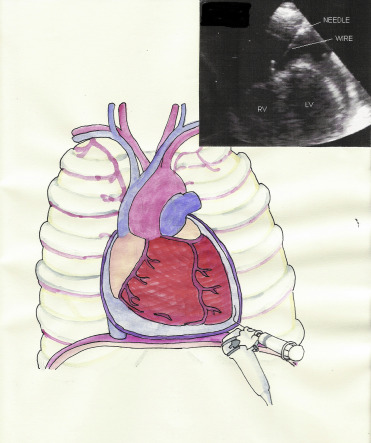
When the needle tip was observed on the echo screen in the pericardial space ( Figure 2 ), and fluid was freely aspirated, the syringe was disconnected, and a j-tipped guide was inserted. The procedure is summarized in Figure 3 ( Movie in online version only). If the drained fluid was bloody, guide insertion was preceded by agitated saline injection to contrast the pericardial fluid and to confirm non-intracardiac insertion. The needle was withdrawn, a small incision was made with a scalpel, and a dilator was inserted. Finally, the dilator was removed, and an 8Fr drainage catheter was inserted along the guidewire. The pericardial effusion was aspirated with a syringe, and the catheter was connected to a disposable flushing system (a large squeeze bulb for a saline 500 ml with a pressure gauge cuffed at 300 mm Hg) that infused saline solution at a rate of 3 ml/hour to maintain the patency of the system. Aspiration by syringe was repeated every 4 to 6 hours. The catheter was left in place until no more than 25 to 30 ml of fluid per day was drained, and echo was negative for significant residual effusion. A complete echocardiographic study was performed in all patients before removing the catheter and before their discharge from the intensive cardiac care unit. To exclude periprocedural complications, in particular pneumothorax or hemorrhage, a chest x-ray was performed at the end of the procedure and blood count was monitored. Fluid samples were drawn for chemical, physical, cultural, and cytologic analyses. In patients without a clear origin of the pericardial effusion, a contrast-enhanced chest tomography was performed.
Results
During the study period, 161 pericardiocentesis procedures were performed in 141 patients (94 men and 47 women). The mean age ± SD for the overall cohort was 62.9 ± 16.0 years (range 18 to 97). The underlying causes of effusion are reported in Table 1 .
| Etiology | (n=141) |
|---|---|
| Malignancy | 48 (34.0 %) |
| Infections: | 16 (11.2 %) |
| Associated pneumonia | 8 (5.6 %) |
| Tuberculosis | 6 (4.2 %) |
| Sepsis by Meningococcus | 1 (0.7 %) |
| Sepsis by Escherichia Coli | 1 (0.7 %) |
| Viral-idiopathic | 16 (12.0 %) |
| Post-cardiotomy syndrome | 14 (9.9 %) |
| Associated to OAC ∗ | 8 (5.6 %) |
| Iatrogenic | 6 (4.2 %) |
| Post-myocardial infarction | 6 (4.2 %) |
| Rheumatologic disease | 5 (3.5 %) |
| Systemic lupus erythematosus | 1 (0.7 %) |
| Rheumatoid arthritis | 1 (0.7 %) |
| Churg Strauss disease | 1 (0.7 %) |
| Mixed connective tissue disease | 1 (0.7 %) |
| Systemic sclerosis | 1 (0.7 %) |
| Uremia | 7 (4.9 %) |
| Heart failure | 2 (1.4 %) |
| Cirrhosis | 2 (1.4 %) |
| Chronic idiopathic | 5 (3.5 %) |
| Pericardial cyst | 1 (0.7 %) |
| Cholesterol (“gold paint” pericarditis) | 1 (0.7 %) |
| Polytrauma | 1 (0.7 %) |
| Gun shot | 1 (0.7 %) |
| Aneurism rupture | 1 (0.7 %) |
| Hypothyroidism | 1 (0.7 %) |
An apical or para-apical puncture was performed in most cases (69%). Alternative routes were subxiphoid in 43 patients (26.7%), left parasternal in 2 cases (1.2%), and right apical approach in 1 patient (0.6%) with dextrocardia (the procedure was carried out in the same fashion with no particular tricks). The puncture site was not reported in 4 patients (2.5%).
In most cases (84%), the procedure was carried out by 2 physicians (1 who performed the echocardiogram and another who performed the puncture and drainage). In 1 case, the probe was held by a nurse and the pericardiocentesis was performed by a physician. In 24 cases, the entire procedure was performed by a single physician.
The procedure was performed for cardiac tamponade in 128 patients, for large pericardial effusion (defined as the sum of anterior and posterior echo-free spaces >20 mm at end-diastole) in 29 cases and for diagnosis in 4 patients with moderate effusions. The diagnosis of cardiac tamponade was based on clinical (tachycardia, dyspnea, hypotension, and pulsus paradoxus) and echocardiographic parameters (right ventricular diastolic collapse, right atrial collapse, and inspiratory decrease in mitral E wave velocity of ≥25%).
The amount of fluid obtained at the initial aspiration ranged from 20 to 2.000 ml (the mean ± SD was 815.08 ± 480 ml); in the following days, we drained 400.0 ± 141.4 ml. After the drainage, the effusion was classified as large if >400 ml of fluid was obtained by initial aspiration (n = 131 patients), moderate if the fluid obtained was between 100 and 400 ml (n = 20 patients), and mild if the fluid obtained was <100 ml (n = 10 patients). The characteristics of the effusion are listed in Table 2 .
| Type of Effusion | Number of Cases | Percentage |
|---|---|---|
| Bloody | 84 | 52.2 % |
| Serosanguinous | 31 | 19.2 % |
| Serous | 39 | 24.2 % |
| Purulent | 1 | 0.6 % |
| Not reported | 6 | 3.8 % |
| Total | 161 | 100 % |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


