Enzyme replacement therapy has the potential to delay or reverse adverse cardiac remodeling in Anderson-Fabry disease (AFD); however, the current indications for enzyme replacement therapy rely on detecting relatively advanced features of the disease. We aimed to determine the relation between the serum N-terminal pro-brain natriuretic peptide (NT-proBNP) concentration and cardiac abnormalities in patients with AFD. We hypothesized that it might help to detect early disease. NT-proBNP was measured under at rest conditions in 117 patients with AFD (age 48 ± 15 years, 46.2% men). All patients underwent clinical evaluation with electrocardiography and echocardiography. The median NT-proBNP concentration was 24 pmol/L (range <5 to 6,059). Of the 117 patients, 67 (57%) had elevated, age-corrected, NT-proBNP levels. In the 56 patients (48%) with normal echocardiographic findings, the NT-proBNP levels were greater than the age-predicted cutoffs in 10 of 25 patients with abnormal electrocardiographic findings and 3 of 31 patients with normal electrocardiographic findings (p <0.05). On multiple regression analysis, age, creatinine, left atrial volume index, E/Ea, and the presence of abnormal electrocardiographic findings were independently associated with log NT-proBNP (R 2 = 0.67, p <0.05). In conclusion, NT-proBNP concentrations were elevated in patients with AFD and early cardiac involvement, suggesting its measurement could assist in decisions regarding the timing of enzyme replacement therapy.
Anderson-Fabry disease (AFD) is an X-linked lysosomal storage disorder, caused by deficiency of α-galactosidase A. This leads to accumulation of glycosphingolipid in tissues throughout the body and subsequent organ failure. In the heart, this typically manifests as left ventricular hypertrophy, which can progress to systolic and diastolic heart failure. Enzyme replacement therapy (ERT) has the potential to delay or reverse adverse cardiac remodeling. However, the current indications for ERT rely on the detection of relatively advanced features of the disease, when irreversible organ damage may have occurred. Brain natriuretic peptide is a cardiac neurohormone secreted from the ventricles of the heart in response to increased wall stress. Brain natriuretic peptide and the N-terminal fragment of its pro-hormone (NT-proBNP) have an established role in determining the diagnosis and prognosis of heart failure. In a male mouse knockout model for Fabry’s disease, the brain natriuretic peptide mRNA levels were significantly increased compared to that of wild-type controls, despite a mild cardiac phenotype. NT-proBNP has recently been associated with overall disease severity in AFD ; however, its role as a marker of early cardiac involvement has not been examined. The primary aim of the present study was to determine the relation between NT-proBNP levels and conventional markers of cardiac involvement in AFD.
Methods
This was an observational, cross-sectional cohort study. Data were collected prospectively. The cohort consisted of consecutively evaluated patients with AFD, seen at a dedicated cardiomyopathy clinic from April 2008 to April 2011. The diagnosis of AFD was determined by measuring the plasma and/or leukocyte α-galactosidase A enzyme activity, followed by sequencing of the α-galactosidase A gene. All patients were aged ≥18 years at evaluation. The study conformed to the principles of the Helsinki declaration.
All patients were evaluated clinically using a 12-lead electrocardiogram at rest and echocardiography. The New York Heart Association class, current medication, and blood pressure were recorded. The Mainz severity score index was used as an index of overall disease severity. Serum creatinine was used to estimate the glomerular filtration rate using the 4-variable Modification of Diet in Renal Disease equation. NT-proBNP was measured from routine venous blood samples taken at rest.
The venous blood samples were drawn into serum separator tubes and sent to the laboratory for quantification the same day as routine sample analysis. Serum NT-proBNP was measured using a 2-site electrochemiluminescence immunoassay on a Roche E170 analyzer. The results are reported in pmol/L, with a lower limit of detection of 5 pmol/L. The manufacturer’s guidelines, derived from 1,981 blood donors aged 18 to 65 years and 283 subjects with no known cardiac disease aged 50 to 90 years, were used to define normal values ( Supplemental file S1 ). Values greater than the 97.5th percentile for age and gender were considered abnormal.
Standard 12-lead electrocardiograms were recorded at 25 mm/s and 10 mm/mV. The rhythm, heart rate, PR interval duration, and QRS complex duration were measured in milliseconds. A QRS duration >120 ms, PR interval <120 ms, or PR interval >200 ms was considered abnormal. Left ventricular (LV) hypertrophy was assessed using the Sokolow-Lyon and Cornell criteria. Patients were considered to have electrocardiographic (ECG) evidence of LV hypertrophy if they met 1 or both criteria. Repolarization changes were assessed in 3 regions: inferior (leads II, II, and aVF), anterior (leads V 1 to V 4 ), and lateral (leads V 5 to V 6 , I, and aVL). Patients with T-wave inversion in ≥1 of these regions were classified as having repolarization abnormalities. Abnormal ECG findings were defined as ≥1 of the following: conduction disease (abnormal QRS duration or PR interval); repolarization changes; and LV hypertrophy according to the voltage criteria.
Patients with a paced rhythm were grouped according to ECG abnormalities present before pacemaker implantation.
Transthoracic echocardiography was performed with the patient in the left lateral decubitus position with commercially available equipment (M3S Probe, Vivid i or Vivid 7; GE-Vingmed, Horten, Norway). The images were stored digitally for off-line analysis (EchoPac, version 108.1.5; GE-Vingmed). Complete 2-dimensional, color, pulsed, and continuous wave Doppler images were acquired using standard techniques. The LV ejection fraction was calculated using Simpson’s biplane method. The left atrial volumes were calculated using the ellipsoid model and indexed the to body surface area (left atrial volume index). The relative wall thickness was calculated as (interventricular septal thickness at diastole + posterior wall thickness in diastole)/LV end-diastolic diameter and is expressed as a percentage. The LV mass was calculated using the Devereux cubed formula (0.8 × {1.04 × [(LV end-diastolic diameter + posterior wall thickness in diastole + interventricular septal thickness at diastole) 3 −(LV end-diastolic diameter) 3 ]} + 0.6 g) and indexed to the body surface area to obtain the LV mass index. Patients with a normal LV mass index were classified as having concentric remodeling in the presence of a relative wall thickness >42% or normal geometry if the relative wall thickness was ≤42%. Patients with an increased LV mass index were classified as having concentric hypertrophy if the relative wall thickness was >42% or eccentric hypertrophy if the relative wall thickness was ≤42%. Diastolic dysfunction was graded according to the mitral inflow pattern, pulmonary vein flow, and tissue Doppler indexes at the lateral mitral annulus and classified as normal, mild (impaired relaxation), moderate (pseudonormal), or severe (restrictive). An estimate of LV filling pressure was made using the ratio between transmitral peak E velocity and the peak Ea velocity measured at the lateral wall (E/Ea). The Tei LV performance index was calculated using pulsed wave spectral Doppler traces of the LV outflow tract and transmitral inflow. The LV outflow tract gradients were measured at rest and after provocation with the Valsalva maneuver. LV outflow tract obstruction was defined as a gradient of ≥30 mm Hg. In the present study, abnormal echocardiographic findings were defined as either interventricular septal thickness at diastole or posterior wall thickness in diastole of ≥13 mm or LV mass index of ≥95 g/m 2 in women and ≥115 g/m 2 in men.
The data were analyzed using PAWS statistical software, version 18.0. Continuous variables are presented as the mean ± SD and categorical variables as frequencies and percentages. Logarithmic transformation allowed the NT-proBNP concentration to be treated as a normally distributed variable. Bivariate correlation analysis was performed using linear regression. To identify independent correlates of logNT-proBNP, all variables with p <0.05 on univariate analysis were included in a forward elimination multivariate regression analysis. Receiver operating characteristics curve analysis was performed to test the ability of NT-proBNP to detect cardiac involvement, defined by either abnormal ECG or echocardiographic findings. Fisher’s exact test was used to calculate the significance of the observed data. The 95% confidence intervals were 2-sided with an α level of 5%.
Results
During a 3-year period, 117 patients were studied: 54 (46.2%) were men, with a mean age of 48 ± 15 years. The clinical and echocardiographic characteristics of the study population are listed in Table 1 . The median NT-proBNP concentration was 24 pmol/L (range <5 to 6,059). Of the 117 patients, 67 (57%) had elevated, age-corrected, NT-proBNP levels. Three patients had an NT-proBNP concentration >1,000 pmol/L, two with end-stage renal failure, who required hemodialysis (6,063 and 4,139 pmol/L), and one with advanced mesothelioma (1,487 pmol/L). These patients were considered outliers and were excluded from the subsequent correlation analysis.
| Clinical Characteristics | Value |
|---|---|
| Male gender | 54 (46%) |
| Age (yrs) | |
| Mean ± SD | 48 ± 15 |
| Range | 19–79 |
| Mainz severity score index | |
| Mean ± SD | 20 ± 11 |
| Range | 1–53 |
| Creatinine (μmol/L) | |
| Mean ± SD | 91 ± 76 |
| Range | 43–692 |
| Glomerular filtration rate (ml/min/1.73 m 2 ) | |
| Mean ± SD | 87 ± 27 |
| Range | 7–158 |
| Hemoglobin (g/dl) | |
| Mean ± SD | 13.2 ± 1.4 |
| Range | 8.9–16.7 |
| Systolic blood pressure (mm Hg) | |
| Mean ± SD | 118 ± 16 |
| Range | 84–165 |
| Diastolic blood pressure (mm Hg) | |
| Mean ± SD | 72 ± 10 |
| Range | 40–91 |
| Heart rate (beats/min) | |
| Mean ± SD | 64 ± 12 |
| Range | 38–93 |
| Medications | |
| Enzyme replacement therapy | 97 (83%) |
| β Blocker | 23 (20%) |
| Angiotensin-converting enzyme inhibitor | 37 (32%) |
| Angiotensin II receptor blocker | 15 (13%) |
| New York Heart Association class | |
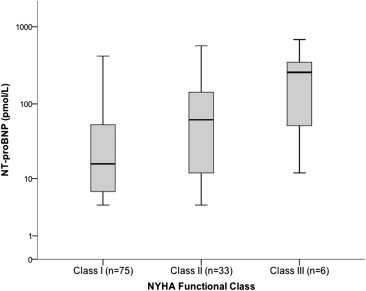
| I | 75 (64%) |
| II | 33 (28%) |
| III | 9 (8%) |
| Rhythm | |
| Sinus rhythm | 102 (87%) |
| Atrial fibrillation | 4 (3%) |
| Paced rhythm | 11 (9%) |
| Atrial fibrillation | |
| Paroxysmal | 13 (11%) |
| Persistent | 4 (3%) |
| Echocardiographic findings | |
| Ejection fraction (%) | |
| Mean ± SD | 61 ± 6 |
| Range | 31–74 |
| Left ventricular end-diastolic diameter (mm) | |
| Mean ± SD | 48 ± 5 |
| Range | 36–61 |
| Left ventricular mass index (g/m 2 ) | |
| Mean ± SD | 113 ± 45 |
| Range | 49–332 |
| Left atrial size (mm) | |
| Mean ± SD | 39 ± 7 |
| Range | 27–58 |
| Left atrial volume index (ml/m 2 ) | |
| Mean ± SD | 25 ± 10 |
| Range | 12–60 |
| Left ventricular outflow tract obstruction | 3 (3%) |
| Remodeling | |
| Normal | 47 (40%) |
| Concentric | 16 (13%) |
| Concentric hypertrophy | 45 (39%) |
| Eccentric hypertrophy | 9 (8%) |
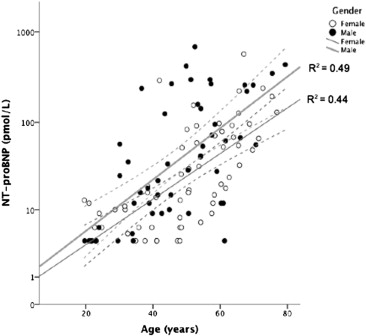
The NT-proBNP concentrations were greater in the men and correlated with age ( Figure 1 ). The values in patients not receiving ERT (n = 20) ranged from <5 to 1,487 pmol/L. The NT-proBNP concentration also increased according to the New York Heart Association class ( Figure 2 ) and grade of diastolic dysfunction ( Figure 3 ). The LogNT-proBNP correlated with the Mainz severity score index score, systolic blood pressure, serum creatinine, and echocardiographic indexes of LV function (E/Ea and Tei index; Table 2 ). No relation was seen between LogNT-proBNP and LV size, ejection fraction, or LV outflow tract obstruction. In a multivariate linear regression model, LogNT-proBNP independently correlated with age, serum creatinine, left atrial volume index, the E/Ea ratio, and abnormal ECG findings (R 2 = 0.67, p <0.05).
| Parameter | Univariate | Multivariate | ||
|---|---|---|---|---|
| β Coefficient | p Value | β Coefficient | p Value | |
| Age (yrs) | 0.656 | <0.0001 | 0.265 | 0.001 |
| Male gender | 0.129 | 0.171 | ||
| Mainz severity score index | 0.514 | <0.0001 | — | NS |
| Systolic blood pressure (mm Hg) | 0.170 | 0.07 | ||
| Glomerular filtration rate (ml/min/1.73 m 2 ) | −0.585 | <0.0001 | −0.188 | 0.012 |
| Abnormal electrocardiographic findings | 0.518 | <0.0001 | 0.155 | 0.024 |
| Ejection fraction (%) | 0.013 | 0.887 | ||
| Left ventricular end-diastolic diameter (mm) | −0.118 | 0.213 | ||
| Left atrial volume index (mls/m 2 ) | 0.610 | <0.0001 | 0.272 | <0.001 |
| Left ventricular mass index (g/m 2 ) | 0.616 | <0.0001 | — | NS |
| Right ventricular hypertrophy | 0.437 | <0.0001 | — | NS |
| E/Ea | 0.642 | <0.0001 | 0.236 | 0.002 |
| E/A ratio | −0.288 | <0.0001 | — | NS |
| Tei index | 0.292 | 0.002 | — | NS |
| Mitral regurgitation | 0.365 | <0.0001 | — | NS |
| Left ventricular outflow tract obstruction | 0.179 | 0.056 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


