Fig. 5.1
Human cells are found in the lungs of bleomycin-treated mice after transplantation with hES cells differentiated into lung lineage-specific cells. 3,3′-Diaminobenzidine (DAB) immunocytochemistry using anti-human nuclear factor antibody was employed to detect human cells in the lungs of bleomycin-treated Rag2γC−/−mice transplanted with lung lineage-specific differentiated H7 hES cells (a–d). The transfer of the human cells was confirmed by qPCR of human Alu element sequence (e–i), and in situ hybridization with human pan-centromeric probe (j–m). The treatment groups consisted of mice given 105 H7 hES cells differentiated in SAGM alone (SAGM group; c, h, l) or with 5 μM ICG-001 (SAGM + ICG-001 group; d, i, m). The control groups consisted of either untreated mice (f, j) or bleomycin-treated mice (Bleo/Saline group; b, g, k). The positive control of H7 hES cells (a, e) is also shown. Human nuclear-specific antibody staining (a–d) and DAB-positive pan-centromeric probe reactions (l, m) are indicated by the brown staining; dissociation curves are shown for the qPCR reaction using the Alu-specific primer (e–i). Figure and legend reprinted with permission by PLoS One (Banerjee ER, Laflamme MA, Papayannopoulou T, Kahn M, Murry CE, Henderson WR Jr (2012). Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One 7 (3):e33165. doi: 10.1371/journal.pone.0033165. Epub 2012 Mar 28)
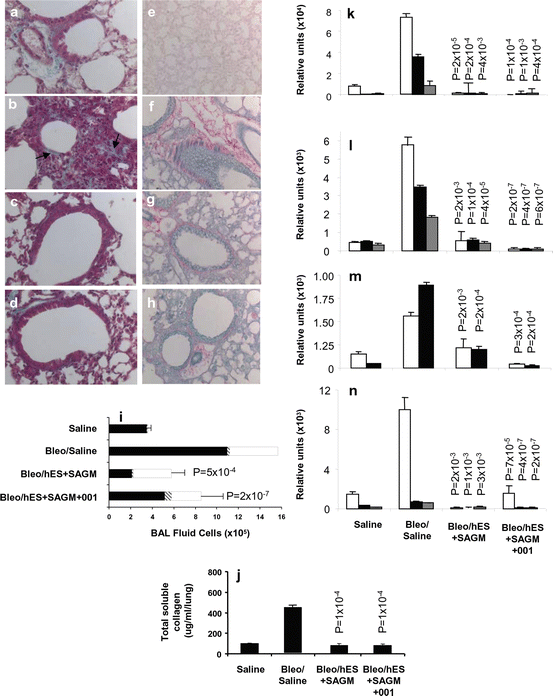
Fig. 5.2
Transplantation of lung lineage-specific differentiated hES cells reverses bleomycin-induced lung inflammation and fibrosis. Transplant groups consisted of Bleomycin-treated Rag2γC−/− mice transplanted intratracheally with 105 H7 hES cells differentiated in SAGM alone (Bleo/hES + SAGM group; c, g, i–n), or presence of 5 μM ICG-001 (Bleo/hES + SAGM + ICG-001 group; d, h, i–n). Control groups consisted of saline-treated control Rag2γC−/− mice (Saline group; a, e) and bleomycin-treated Rag2γC−/− mice given saline intratracheally (Bleo/Saline group; c, g, i–n). Collagen was detected by Masson’s trichrome (a–d) and Picro Sirius red (e–h) stains. BAL fluid cell counts (i) are shown for macrophages (solid bars), lymphocytes (hatched bars), and neutrophils (open bars). Total soluble collagen/lung was measured by the Sircol™ assay (j). qPCR was used to determine differential expression in the lungs of the following genes: collagen 1α2 (white bars), collagen 3α1 (black bars), and collagen 6α1 (gray bars) (k); transforming growthFig. 5.2 (continued) factor-β1 (white bars), transforming growth factor-β2 (black bars), and transforming growth factor-β3 (grey bars) (l); fibroblast growth factor-1 (white bars) and fibroblast growth factor-2 (black bars) (m); and vascular endothelial growth factor-A (white bars), vascular endothelial growth factor-B (black bars), and vascular endothelial growth factor-C (gray bars) (n). P < 0.05 values compared to bleomycin-treated control group administered saline are shown. Figure and legend reprinted with permission by PLoS One (Banerjee ER, Laflamme MA, Papayannopoulou T, Kahn M, Murry CE, Henderson WR Jr (2012) Human embryonic stem cells differentiated to lung lineage-specific cells ameliorate pulmonary fibrosis in a xenograft transplant mouse model. PLoS One 7 (3):e33165. doi: 10.1371/journal.pone.0033165. Epub 2012 Mar 28)
The resolution of bleomycin-induced airway fibrosis in the mice receiving the human stem cell transplants was accompanied by an expansion in progenitor numbers in the bone marrow, blood, spleen, bronchoalveolar (BAL) fluid, and lung and increase in both AT1 and AT2 cells that were not observed in control mice. ICG-001 treatment of the cells differentiated in SAGM in vitro expanded the Club cell population after engraftment and caused a further marked increase in the number of lung progenitors, suggesting that modification of the cells in vitro by this specific inhibitor of Wnt/β-catenin/CBP-signaling resulted in a persistent effect on progenitor number in the lungs (Banerjee et al. 2012). These data in a mouse IPF model indicate that progenitor cells from both hematopoetic (i.e., bone marrow) and non-hematopoetic (i.e., lung, splenic lymphoid tissue) niches circulate to the lungs after local injury where they contribute to the resolution of the ensuing pulmonary fibrosis.
5.2.5 Stem Cell Transplantation in Patients with IPF
Clinical research studies to investigate the safety and potential efficacy in ameliorating lung fibrosis in IPF patients have recently been initiated (Kørbling and Estrov 2003). The stromal vascular fraction (SVP) within the adipose tissue contains adipose stromal cells (ADSCs) that, in culture, acquire immunophenotypic characteristics of bone marrow-derived MSCs and reduce lung fibrosis in animal models. In a Phase 1b, non-randomized, non-placebo-controlled prospective unicentric study to assess safety, autologous ADSCs-SVF were administered endobronchially in 14 IPF patients of mild-moderate severity (Tzouvelekis et al. 2013). The stem cell infusions (three at monthly intervals; 0.5 × 106 cells/kg body weight/infusion) were well-tolerated with no clinically significant adverse reactions observed. Of note, no ectopic tissue was observed by whole body CT scan 12 months after the first endobronchial infusion in the study group. Retention of hexametazine 99mTc-HMPAO radiolabeled cells within the lungs for 24 h after the endobronchial delivery was observed; further tracking of the cells was not possible due to the inability to produce signal greater than 24 h after infusion (Tzouvelekis et al. 2013).
MSCs derived from term placenta have been employed in a Phase 1b non-randomized, dose escalation study in patients with IPF of moderate severity to test the safety of this potential therapeutic approach (Chambers et al. 2014). In this single-center study, eight patients received either 1 × 106 MSCs/kg (n = 4) or 2 × 106 MSCs/kg (n = 4) intravenously and were followed for a 6-month period for adverse events and lung function. Short-term safety of the infusions was found with one subject having possible embolization of the MSCs to an impaired pulmonary vascular bed, but rapid resolution of the resulting minimal hemodynamic and gas exchange decrements. At 6 months after MSC infusion, lung function and high-resolution chest CT scores were unchanged from baseline. Pulmonary disease worsening was observed in two of the eight subjects, but occurred approximately at 176 days and 6 months after the stem cell transplants. It is unknown whether this decline in lung function was related to the MSC transplants since some studies have suggested that MSCs contribute to the profibrotic process in lung injury (Walker et al. 2011) or rather consistent with the natural history of the rapid decline in lung function observed in this uniformly fatal lung disorder that has a median survival period of less than 4 years. Thus, initial steps in stem cell transplantation have not exhibited significant toxicity and set the stage for prospective, controlled multi-center studies to confirm the safety of this therapeutic intervention and assess its efficacy in resolving lung fibrosis and reversing or preventing further decline in lung function in IPF patients.
5.2.6 Characterization of Lung Stem Cell Niches
Stem cell niches are protected tissue sites where stem cells reside and mobilize in response to local injury (Watt and Hogan 2000; Spradling et al. 2001; Hong et al. 2001; Lynch and Engelhardt 2014). The tissue stem cells have an undifferentiated phenotype and rarely proliferate. The lung stem cell niches have been examined using rodent models of epithelial denudation and recovery (Hong et al. 2001, 2004; Londhe et al. 2011). Bronchiolar Club cell progenitors are critical for the restoration of alveolar epithelial cells, bronchiolar, and bronchial cells after airway damage. For example, deletion of Club cell progenitors by ganciclovir treatment of transgenic mice expressing HSV-TK driven by a Club cell-specific promoter (CCSP) prevents reconstitution of the normal alveolar and bronchiolar epithelium (Hong et al. 2001; Londhe et al. 2011). Ciliated cells in the airways that survive injury induced by naphthalene and other toxic agents release Wnt7b that induces in the parabronchial smooth muscle cell niche expression of fibroblast growth factor 10 (Fgf10). Fgf10 is important for the maintenance of the distal airway epithelial progenitor cells (Volckaert et al. 2011; Volckaert and De Langhe 2014). The Fgf10-mediated activation of the parabronchial smooth muscle cell niche is regulated by the Wnt target c-Myc; ablation of c–Myc impairs regeneration of the airway epithelium after injury (Volckaert et al. 2013; Volckaert and De Langhe 2014).
A basal cell population expressing cytokeratin 14 has a multipotent differentiation capacity for repair of airway epithelium (Hong et al. 2001). Stem cell niches in the tracheal and proximal conducting airways have been identified by their resistance to injury with sulfur dioxide and the detergent polidocanol (Borthwick et al. 2001). Bronchioalveolar stem cells (BASCs) are naphthalene-resistant cells located at the junction between the conducting and respiratory epithelium in the bronchoalveolar duct junction (BADJ) in terminal bronchioles (Giangreco et al. 2002; Kim et al. 2005; Summer et al. 2003). Club cells can be restored by naphthalene-resistant stem cells associated with neuroepithelial bodies in proximal airways (Kim et al. 2005). The BADJ-associated Sca1+ CD34+ cluster of differentiation 31 (CD31)− CD45− cell population is dual-positive for both pro-SPC and CCSP (Kim et al. 2005).
The bleomycin mouse model of pulmonary fibrosis has also been employed to characterize lung stem cell niches. In these studies, the DNA analogue bromodeoxyuridine (BrdU) was administered intraperitoneally after intratracheal delivery of bleomycin in pulse chase experiments to identify label-retaining cell (LRC) BrdU+ stem cells (Banerjee and Henderson 2012b). BrdU+ LRCs were found in both luminal and basal locations throughout the trachea within the first week of lung injury induced by bleomycin and were later observed in submucosal gland ducts in the proximal trachea and also near the cartilage–intercartilage junction of the distal trachea. Contributing to regeneration of bleomycin-damaged lung in this mouse model were progenitors from the hematopoietic pool in the lungs (Banerjee and Henderson 2012b).
5.3 Allergen-Induced Airway Remodeling in Asthma
Airway remodeling is a characteristic feature in patients with asthma with structural changes beginning in early childhood (Lazaar and Panettieri 2003; Holgate et al. 2003; Payne et al. 2003). Key structural changes include airway wall thickening, goblet cell metaplasia with mucus hypersecretion, smooth muscle hyperplasia, angiogenesis, and subepithelial fibrosis (Henderson et al. 2002, 2006; Banerjee and Henderson 2013). Th2 cytokines (i.e., interleukin-4 (IL-4), interleukin 5 (Il-5), interleukin 13 (IL-13)), and cysteinyl leukotrienes (CysLTs) C4, D4, and E4 play an important role in the immunopathogenesis of the remodeling (Holgate et al. 2003; Henderson et al. 2002, 2006). IL-13 and the growth factor TGF-β are most closely associated with the development of the airway fibrosis and altered lung function and hyperresponsiveness. TGF-β and IL-13 increase expression of the cysteinyl1 receptor (CysLT1R) for cysteinyl leukotriene (CysLT)s C4, D4, and E4 on human airway smooth muscle cells, which is likely important in the thickening of the airway smooth muscle layer since CysLTs augment epidermal growth factor (EGF)-induced proliferation of human airway smooth muscle cells (Mehrotra and Henderson 2009). An excess decline in lung function is observed in both children and adult patients with these features of chronic asthma and who have frequent recurrent severe exacerbations that are refractory to corticosteroid therapy (The Childhood Asthma Management Program Research Group 2000; Covar et al., 2004; Guilbert et al. 2006; Bai et al. 2007).
Thickening of the airway wall occurs from deposition of ECM proteins such as collagen, tenascin, fibronectin, and laminin and proteoglycans such as lumican, biglycan, and versican in the subbasement membrane lamina reticularis (Huang et al. 1999; Christie et al. 2004). Spatial differences have been observed in asthmatic airways in the production of ECM components with greater versican production in distal compared to central airway fibroblasts (Nihlberg et al. 2010). In patients with mild asthma, the thickening of the basement membrane correlates the number of tissue fibrocytes suggesting that fibroblast progenitor cells may play a major role in the airway remodeling process (Nihlberg et al. 2006). After bronchial allergen challenge, fibrocytes localize to the bronchial submucosa in patients with allergic asthma (Schmidt et al. 2003). The circulating fibrocytes from allergic asthmatics have increased levels of the interleukin-33 (IL-33) receptor component ST2L compared to non-asthmatic subjects (Bianchetti et al. 2012). Both fibrocyte chemotaxis and proliferation are stimulated in vitro by the epithelial-derived cytokine IL-33 (Bianchetti et al. 2012), providing a link between allergen-induced airway injury with fibrocyte infiltration in asthma.
Increased circulating CD34+CD45+/collagen-1+ fibrocytes are found in asthmatic patients who have chronic airflow obstruction compared to those without airflow obstruction (Wang et al. 2008). In vitro, fibrocytes from these subjects with chronic airflow obstruction can be transformed by TGF-β1 into myofibroblasts, critical cells for the remodeling process (Wang et al. 2008). Proteomic analyses of BAL fluid from patients with mild asthma have demonstrated that increased expression levels of the acute phase glycoprotein haptoglobin occur in conjunction with differentiation of fibrocytes into fibroblast-like cells (Larsen et al. 2006). These data suggest a potential role for haptoglobin in trafficking fibroblast progenitor cells to the airways and promoting their differentiation into activated fibroblasts, key for the increased production of ECM proteins in the thickening airways. In patients with mild-to-refractory asthma, there is a marked increase in the number of fibrocytes in their airway smooth muscle bundles compared to healthy controls (Saunders et al. 2009). Fibrocyte chemotaxis and chemokinesis are stimulated in part by platelet-derived growth factor (PDGF) released by airway smooth muscle cells (Saunders et al. 2009). Induced sputum from patients with severe asthma compared to patients with less-severe and treatment-responsive asthma contain increased levels of the chemokine (C–C motif) ligand 5 (CCL5), chemokine (C–C motif) ligand 11 (CCL11), and chemokine (C–C motif) ligand 24 (CCL24) that promote fibrocyte chemotaxis (Isgrò et al. 2013).
Accompanying the thickening of the subendothelial basement membrane and airway wall is an increase in the vascular bed and arteriolar fibrosis (Salvato 2001). The increased number of vessels occurs in both the medium and small airways of asthmatics with a marked increase in the number of angiogenic factor-positive cells compared to normal subjects (Hashimoto et al. 2005). The elevated levels of the angiogenic cytokine VEGF found in the BAL fluid of asthmatics (as compared to non-asthmatic subjects) correlate with the increased expression of VEGF and hypoxia-inducible factor-1α (HIF-1α) and hypoxia-inducible factor-2α (HIF-2α) in the airway submucosa in these patients with asthma (Lee et al. 2006). HIF-1α that induces the transcriptional response to hypoxia regulates progenitor cell trafficking to injured tissues through induction of the chemokine SDF-1 (Ceradini et al. 2004; Ceradini and Gurtner 2005).
The interaction of allergens that contain innate immune-activating components with mannose receptor (MRC1; cluster of differentiation 206 (CD206)), a C-type lectin receptor found on fibrocytes, has recently been explored. Epidemiologic studies in New York City have found that the cockroach allergen Bla g 2 was more often found in home bed dust in neighborhoods with a high asthma prevalence than those with a low asthma prevalence (Olmedo et al. 2011). Bla g 2 binds to CD206 and is taken up by human fibrocytes that express CD206 in a process blocked by anti-human mannose receptor antibody (Tsai et al. 2013). Bla g 2-induced cytokine (i.e., TNF-α and interleukin 6 (Il-6)) secretion and nuclear factor kappa-B (Nf-κB), extracellular signal-regulated kinase (ERK), and Jun amino-terminal kinase (JNK) activation in the fibrocytes were also mediated via CD206 (Tsai et al. 2013). These data suggest that innate pattern recognition interaction between cockroach allergens and CD206 in circulating fibrocytes can modulate allergic responses in the airways.
Because airway remodeling and the loss of lung function in asthmatics are not reversed by corticosteroids or other therapies, there is intense interest in discovery of new approaches to reversing this process; one such approach is stem cell-based intervention. Mouse asthma models that replicate key features of Th2 cytokine/TGF-β-driven airway remodeling have recently been used to further understand the role of progenitor cells in this process. In these models, there is allergen-induced recruitment of T cell, macrophage, neutrophil, and basophil progenitors as well as stem cells to the lungs and BAL fluid (Banerjee and Henderson 2012a, 2013; Gao et al. 2014).
Induced pluripotent stem cells (iPSCs) may serve as a source for multilineage differentiation and proliferation. iPSCs reprogrammed from adult somatic cells that were transfected by Oct-4/Sox-2/Klf-4 but not by c-Myc were investigated in an ovalbumin (OVA) allergen-driven mouse asthma model with eosinophilia for their modulatory effect on airway inflammatory responses (Wang et al. 2013). In this Th2 cytokine-mediated model, Th2 antibody responses, IL-5 levels in the BAL fluid, and airway hyperresponsiveness of the OVA-treated mice were significantly decreased by the administration of iPSCs. Intranasal administration of miRNA-reprogrammed iPSCs that do not express oncogenic transcription factors such as c-Myc and Kruppel-like factor-4 (Klf-4) has also been demonstrated to enhance T regulatory (Treg) cell expansion and IL-10 cytokine release in the lungs and reduce airway remodeling in mice with Th2 cytokine-driven airway inflammation (Ogulur et al. 2014).
Infusion of mouse (Nemeth et al. 2010) and human (Bonfield et al. 2010) MSCs inhibits allergen-induced levels of IgE and the Th2 cytokines IL-4, IL-5, and IL-13 in BAL cells consistent with a dampening of the Th2 asthma phenotype. MSCs derived from adipose tissue infiltrate the lungs after intravenous administration in a mouse model of airway remodeling induced by house dust mite stimulation (Mariñas-Pardo et al. 2014). In this model, reduction in airway inflammation by the MSCs was associated with an initial increase in T-helper type 1 (Th1) chemokines interferon-γ (IFN-γ) and interleukin 12 (IL-12), suggesting a skewing toward a Th1 immune response and away from a pro-allergic Th2 response (Mariñas-Pardo et al. 2014). In a cockroach allergen-induced mouse asthma model, TGF-β promotes migration of MSCs to the airways in a process blocked by TGF-β-neutralizing antibodies (Gao et al. 2014). Similarly, tumor-derived MSCs administered intravenously in an OVA-driven model of allergic asthma inhibit the Th2 phenotype by increasing lung production of TGF-β1 (Song et al., 2014).
In a mouse model of T-helper type 17 (Th17) cytokine-mediated neutrophilic allergic airway inflammation employing Aspergilllus fumigatus as the allergen, administration of syngeneic MSCs decreased airway hyperresponsiveness and Th17-induced inflammation in the lungs (Lathrop et al. 2014). In dust mite-driven airway remodeling in mice, HIF-1α inhibition reduces eosinophilic airway inflammation and recruitment of EPCs to the lungs by decreasing vascular endothelial growth factor-A (VEGF-A) and chemokine (C-X-C motif) ligand 1 (CXCL1) levels in the lungs (Byrne et al. 2013). Dust mite-induced proliferation of peripheral blood mononuclear cells from patients with allergic asthma, but not from allergic individuals without asthma, is inhibited by MSCs (Kapoor et al. 2012). These collective data suggest that Th2 cytokine-induced airway eosinophilic and neutrophilic inflammatory and remodeling responses may be modulated by stem cell therapies.
5.4 Conclusions
There has been an explosion in our knowledge of the role of progenitors in the restoration of lung cells and architecture after airway injury. Circulating progenitors of both hematopoietic and non-hematopoietic origin and local tissue stem cells mobilize and move to the sites of injury in the airways in response to specific signals such as chemokines. Animal models have demonstrated the great potential for administration of multipotent stem cells to reduce the fibrogenic and other airway remodeling responses observed in patients with of IPF and chronic asthma who have aberrant repair. Great excitement exists for future clinical research studies to explore the therapeutic potential of stem cell transplants in patients with progressive lung disorders.
References
Aguilar S, Scotton CJ, McNulty K, Nye E, Stamp G, Laurent G, Bonnet D, Janes SM (2009) Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One 4(11):e8013. doi:10.1371/journal.pone.0008013 CrossRefPubMedCentralPubMed
Andersson-Sjöland A, de Alba CG, Nihlberg K, Becerril C, Ramírez R, Pardo A, Westergren-Thorsson G, Selman M (2008) Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol 40(10):2129–2140. doi:10.1016/j.biocel.2008.02.012, Epub 2008 Mar 11CrossRefPubMed
Andersson-Sjöland A, Nihlberg K, Eriksson L, Bjermer L, Westergren-Thorsson G (2011) Fibrocytes and the tissue niche in lung repair. Respir Res 12:76. doi:10.1186/1465-9921-12-76 CrossRefPubMedCentralPubMed
Antoniou KM, Papadaki HA, Soufla G, Kastrinaki MC, Damianaki A, Koutala H, Spandidos DA, Siafakas NM (2010) Investigation of bone marrow mesenchymal stem cells (BM MSCs) involvement in idiopathic pulmonary fibrosis (IPF). Respir Med 104(10):1535–1542. doi:10.1016/j.rmed.2010.04.015, Epub 2010 May 18CrossRefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


