RCT
N° patients
Active treatment
Comparator
Time of active treatment
Time to FU angiography (if mandatory)
Max period FU
Primary endpoint
IMPRESS [15]
83
Prednisone
Placebo
45 days
6 months
12 months
Death, myocardial infarction and TVR
OSIRIS [17]
300
Usual-dose of sirolimus
High-dose of sirolimus
Placebo
10 days
6 months
4 years
Angiographic restenosis
CEREA-DES [39]
375
Prednisone
BMS (control)
DES (control)
40 days
NA
4 years
Cardiovascular death, myocardial infarction and TVR
ORAR II [25]
100
Sirolimus
BMS
14 days
9 months
12 months
Angiographic restenosis and late loss
Stojkovic et al. [26]
80
Sirolimus
BMS
30 days
6 months
6 months
Angiographic restenosis and late loss
Cernigliaro et al. [27]
108
Sirolimus
BMS
30 days
6 months
5 years
In-stent restenosis
CREST [29]
705
Cilostazol
DAPT + Placebo
6 months
6 months
6 months
Minimal luminal diameter
DECLARE-long [30]
500
Cilostazol
DAPT
6 months
6 months
9 months
In-stent late loss
DECLARE-diabetes [31]
400
Cilostazol
DAPT
6 months
6 months
9 months
In-stent late loss
ORAR III [32]
200
Sirolimus
Cypher, Taxus, Endeavour
14 days
NA
5 years
Cost-effectiveness
Corticosteriods
The effects of corticosteroids are numerous and of importance is their ability to alter the immune response [33]. The known inhibition of inflammatory cells activation, an essential player in the process of neointimal proliferation and restenosis after stent implantation [33–35], was the basis to evaluate corticosteroids for the reduction of restenosis. Experimental studies showed a reduction in neointimal hyperplasia after a 2-week period of continuous hydrocortisone infusion after an aortic balloon injury, although the initial clinical trials in humans failed to achieve equivalent results. Possible reasons for failure were that they were performed in the pre-stent era or the steroids were given intravenously for a short period of time [14, 36].
Two clinical trials comparing oral prednisone with BMS vs. controls showed positive clinical results [15, 37]. The first one [15], evaluated a selected group of non-diabetic patients with CAD and an elevated C reactive protein (CRP) 72 h after successful PCI with a BMS who were randomized either to high and decremental doses of oral prednisone (n = 41) or placebo (n = 42) for 45 days. The primary clinical endpoint was 12 months event-free survival rate, defined as freedom from death, myocardial infarction (MI) or revascularization; angiographic endpoints were late loss and the restenosis rate at 6 months. Event-free survival at 12 months, was significantly lower with placebo (p = 0.006) due to a higher rate of repeat procedures for restenosis. Angiographic restenosis and late loss were also less with prednisone (p = 0.001 for both).
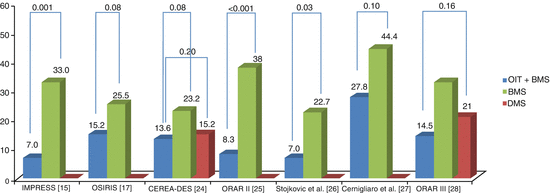
The IMPRESS II/MVD registry [37], included patients with multi vessel disease and showed an event-free survival rate at 12 months of 93 % with prednisone group and 69.8 % with control (p = 0.006) and a significant TVR favorable to the prednisone group (p = 0.01). The pooled data of both studies [38] revealed that, at a mean follow-up of 6.5 ±1.4 years, event-free survival was significantly better with prednisone (87.8 vs. 47.6 %, p < 0.001). The same investigators went further and presented in 2011 and 2012 the CEREA-DES trial [24, 39], where they randomized a larger population to three arms: BMS plus placebo (n = 125), BMS plus prednisone (n = 122) and DES alone (n = 127) groups. Prednisone was given orally for 40 days, 1 mg/Kg the first 15 days after baseline PCI, 0.5 mg/kg/days 16–30 days and 0.25 mg/kg for the last 31–40 days. The primary endpoint was major adverse cardiac events (MACE) at 12 months (death, MI and target vessel revascularization [TVR]). The authors found that patients receiving BMS alone had lower event-free survival versus those treated with prednisone or DES; 80.8 % in controls compared to 88.0 % with prednisone and 88.8 % in DES groups, respectively (P = 0.04 and 0.006). At long term follow-up, patients receiving a BMS alone had significantly lower event-free survival (75.3 %) compared with 84.1 % with prednisone (p = 0.007) and 80.6 % with DES (p = 0.03). DES patients suffered more very late stent thrombosis and MI. The need for TVR remained lower in the prednisone and DES groups (13.6 and 15.2 %, respectively), compared with BMS (23.2 %).
Therefore, in this non-diabetic population, prednisone therapy compared to BMS improved event-free survival at 1 and 4 years. While nearly 15 % of patients suffered minor drug-related side effects, such as facial edema (7 %) and transient hyperglycemia (5 %) none of them discontinued the treatment. A recent pooled data also suggested that prednisone had a significant reduction in restenosis when associated with BMS implantation [40] and may represent a clinical option in non-diabetic patients who are poor candidates for DES.
Cilostazol
Cilostazol is used for the treatment of intermittent claudication. It is a pleiotropic molecule that selectively inhibits the subtype phosphodiesterase 3 (PDE III) that degrades cAMP, causing an accumulation of cAMP within the cell [41], resulting in a direct dilatation of the vascular smooth muscular cell (VSMC). It also acts directly on inhibiting platelet aggregation and VSMC proliferation by inhibiting the mitogen-activated protein kinase (MAPK) and inducing apoptosis via the anti-oncogene p53. There is evidence suggesting that the molecule determines the up-regulation of hepatocyte growth factor (HGF), an endothelial grow factor that accelerates re-endothelization . Also, cilostazol exerts an anti-inflammatory effect by inhibiting leukocyte integrin (MAC-1), which was linked to neointimal thickening and restenosis when its expression and activation during coronary interventions is increased [42]. In summary, its multiple mechanisms of action make it promising for both reducing restenosis and as an antithrombotic agent.
Approved by the FDA, the safety profile of the drug is well established and its pleiotropic characteristics centered attention to its use in CAD, particularly for the prevention of ISR. Data showed a significant reduction in restenosis after PCI in the pre-stent era [18, 19]. With BMS, the CREST trial [29] evaluated cilostazol as a component of triple antiplatelet therapy (TAPT), including aspirin and ADP-receptors antagonists vs. DAPT alone. 705 patients were enrolled and randomized to receive either placebo or cilostazol for 6 months. At follow up angiography, late loss was significantly less with TAPT (p = 0.01) and there was a 52 % reduction in the risk of restenosis.
Subsequently, the DECLARE trials [30, 31] designed to compare DAPT with TAPT in special patient subgroups using DES, also showed favorable results. One of these, the DECLARE-Long Trial, evaluated the impact of 6 months of cilostazol after PCI with DES in patients with lesions >25 mm. The authors randomly assigned 500 patients to DAPT and TAPT groups. At 6 months the primary endpoint of in-stent and in-segment late loss was significantly lower in the TAPT group (p = 0.031 and p = 0.001, respectively). TLR (2.8 % vs. 6.8 %, respectively p = 0.03), and MACE (2.8 % vs. 7.6 %, p = 0.01) were also in favor of TAPT, with a similar incidence of stent thrombosis at 6 months (0.99). In the DECLARE-Diabetes trial [31] they compared TAPT (n = 200) with DAPT (n = 200) for 6 months in diabetics receiving DES and the results again were in favor of TAPT, with significant differences in in-stent and in-segment late loss (p = 0.02 and p = 0.031, respectively). These findings were consistent with the results of a meta-analysis recently published [43] that evaluated 7670 patients from ten controlled clinical trials comparing DAPT with TAPT; they found a significant reduction in ISR using TAPT for 6 months without differences in mortality, MI, TVR and stent thrombosis. In summary, there appears to be an important role for cilostazol in high risk patients, such as diabetics and patients with long lesions and long stents [44].
Sirolimus (Rapamycin)
A macrolide derived from the Streptomyces hygroscopicus, sirolimus is a potent immunosuppressive and antimitotic agent first approved in 1999 to prevent acute rejection of renal transplants [45] and now used in major heart transplant centers to mitigate transplant allograft vasculopathy [46]. It acts by inhibiting the mammalian target of rapamycin (mTOR), blocking cell division in G1 to S1 phases of the mitotic cell cycle thus reducing VSMC proliferation and migration as well as inhibiting extracellular matrix formation [47, 48].
In 1999, the first preclinical data evaluating the use of oral sirolimus after PCI with BMS for the reduction of neointimal thickening in animal restenosis models showed positive results [48]. This lead to studies evaluating the safety and feasibility in pilot trials [49, 50] where patients with de-novo coronary lesions received sirolimus for short periods immediately after BMS. These trials used doses from 2 to 5 mg per day for 14–30 days and both evaluated the incidence of TLR and late loss by angiography at 6 months. The results showed a significant reduction in binary restenosis and the ORAR pilot studies were the first ones that established a significant reduction in both parameters versus BMS alone, when sirolimus serum levels were >8 ng/ml [16, 50]. Subsequently, three RCTs in de-novo coronary lesions confirmed these positive results [25–27].
In 2004, the OSIRIS randomized trial [17] evaluated this approach for the treatment of in-stent restenosis, comparing placebo versus two different loading doses; usual loading dose (8 mg) and high loading dose (24 mg) of sirolimus, both followed by 2 mg per day during 7 days. In accordance with other publications, TVR and restenosis were significantly reduced, with a significant correlation in blood concentration levels on the day of the procedure with late lumen loss reduction at follow-up (p < 0.001).
The Oral Rapamacyn in ARgentina (ORAR) III trial was a randomized comparison between BMS implantation followed by 14 days of rapamycin versus a DES strategy without rapamycin. The DEStents used were Taxus (Boston Scientific Corporation, Natick, MA, USA), Endeavour (Medtronic Vascular, Santa Rosa, CA, USA) and Cypher, (Cordis, Warren, NJ, USA) in 98 % of cases. Patients were followed at 1, 3 and 5 years after PCI [28, 32, 51]. The primary objectives were costs and TVR. Safety was defined as the composite of death, MI and stroke. In the OR arm, patients received a sirolimus-loading dose of 10 mg the day before stent implantation followed by 3 mg per day for 13 days. OR patients received clopidogrel 75 mg a day for 1 month, and DES patients for at least 1 year. Briefly, at 1 and 3 years DES and OR groups had similar safety and efficacy although the OR strategy was cost saving [28, 51]. At 5 years [32], major differences in clinical adverse events between both groups were seen. The incidence of death was 6 % with OR and 16 % with DES (p = 0.02), the composite of death, MI and stroke was 12 % with OR and 25 % with DES (p = 0.01), TLR was 10 % with OR and 17.6 % with DES (p = 0.05) and target vessel revascularization, the composite of cardiac death, MI and target vessel revascularization, 26 % with OR and 36 with DES (p = 0.08) (Fig. 22.1). These differences were driven by poor outcomes with DES in the elderly sub-group of patients. At 5 years, stent thrombosis (definitive/probable/possible) was significantly greater with DES versus OR (9 % vs. 2 %, respectively, p = 0.03). Cumulative cost was higher in the DES group. In conclusion, at 5 years follow-up, the initial DES strategy failed to be cost effective compared to OR plus BMS [32] and this later strategy had a significant reduction in mortality and in the combined end point of death, MI and stroke. The slow progressive deterioration of results with these first generation DES beyond 3 years was observed in other studies. This was in agreement with the findings of ORAR III at 5 years [52–55].
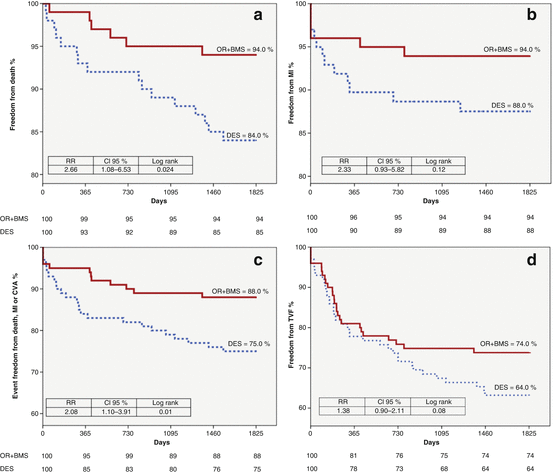

Fig. 22.1
Survival curves for clinical outcomes from 5 years of follow-up from ORAR III randomized clinical trial (OR + BMS vs. first generation DES) (Modified from Rodriguez et al. [32])
Most studies using short treatment protocols of sirolimus (10–14 days) reported low side effects with low rates of discontinuation, around 3 or 4 % [51]. Platelet counts declined transiently with OR therapy but there was no clinical sequellae and they returned to normal after the drug was stopped. A systematic review evaluating the effectiveness of OR after BMS implantation for the prevention of restenosis was published in 2013 [56] including four RCTs in patients with de-novo coronary artery lesions. Three studies were versus BMS alone and the fourth vs. first generation DES. Follow-up duration ranged from 7 months to 5 years and they demonstrated that, compared to BMS alone, early short-term systemic use of OR after BMS led to a significant reduction in both TLR and any adverse cardiac events.
Meta-Analysis
In 2014 a meta-analysis was published [57] using patient level data from all eligible RCTs, using OI therapy with either sirolimus or prednisone for the prevention of restenosis. A total of 1246 patients (608 randomized to BMS plus OI and 638 randomized to BMS/DES and no OI) and 1456 lesions (711 randomized to BMS plus OI and 745 randomized to BMS/DES) were included. In two trials [24, 39], 1 mg/kg/day of prednisone was administered after PCI for 10–15 days with subsequent tapering until complete drug withdrawal after 40–45 days. Five OR RCTs were included [17, 25–28]; in four of them, the loading dose range was 4–24 mg; the other one [26] didn’t use a loading dose. All five RCTs used a daily maintenance dose of 2–3 mg for a period of time lasting from 7 to 30 days after PCI. The primary efficacy outcome was TLR/TVR and the primary safety outcome was the composite of death and MI. Every RCT reported that an independent committee adjudicated clinical endpoints. The meta-analysis found that there was a decrease in the risk of TLR/TVR compared with control arms and, in patients with follow-up angiography, the oral therapy reduced late loss providing a biologic explanation for these results (Figs. 22.2 and 22.3). Moreover, oral treatment didn’t affect the incidence of death and MI compared to controls.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


