Fig. 23.1
Patient positioning with “roll” elevations: the patient is placed on the operating table with the neck and lower legs flexed slightly. Towel ‘rolls’ are placed under the neck, which is turned to the left, right shoulder blade, and right knee. This position widens the intercostal spaces and provides access to both femoral groin regions. The arm is placed by the right side for draping in a sheet sling
Instrument Arm Port Placement
We define and mark the right mid-clavicular and mid axillary lines as well as each rib interspace (Fig. 23.2). After appropriate prepping and draping, the following sites are marked (1) camera port—the 4th interspace (anterior axillary line) (2) working port—4th interspace (posterior to the the anterior axillary line) (3) left robotic arm—3d interspace (mid-axillary line) (4) right robotic arm—5th interspace (posterior axillary line) (5) retractor arm—5th interspace (mid-clavicular to anterior axillary line).
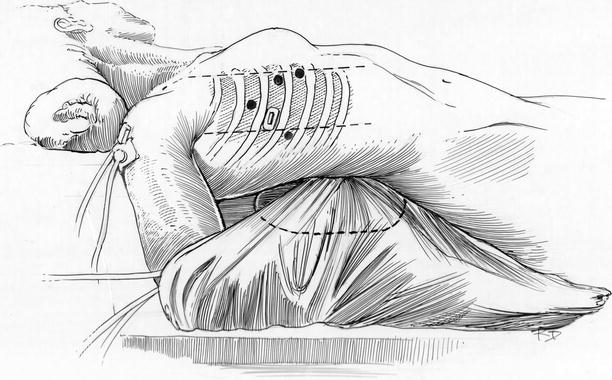

Fig. 23.2
Arm suspension and trocar placement: the right arm is placed in a sheet sling and positioned below the operating table edge. The patient must be moved very close to the table edge so that the arm falls behind the table vertically without impingement. Trocar sites are shown for the right and left instrument arms, the robotic camera and the dynamic retractor. An ATS Medtronic (Medtronic, Inc., St. Paul, MN) rectangular metal trocar is used for the working port
All ports must be placed with the greatest of care to avoid injury to the lung, diaphragm, or liver as well as to provide optimal instrument arm trajectories toward the operative site entrance (superior pulmonary vein and inter-atrial groove). As seen in Fig. 23.3, the camera port is established first and early intra-thoracic vision is used to avoid structural injuries during subsequent port placement. After the 2–3 cm working port is established, the surgeon should place a finger into the thoracic cavity and palpate “safe insertion areas” during subsequent port and pericardial retraction suture placement (Fig. 23.3—Inset). After all ports have been established, the daVinci™ robotic instrument cart (Intuitive Surgical, Sunnyvale, CA) is positioned on the left side of the patient. Instrument arms cross over the table and are directed trough each trocar (Fig. 23.4). Video monitors are oriented to be sure that the tableside surgical assistant has excellent intra-cardiac visualization at all times.
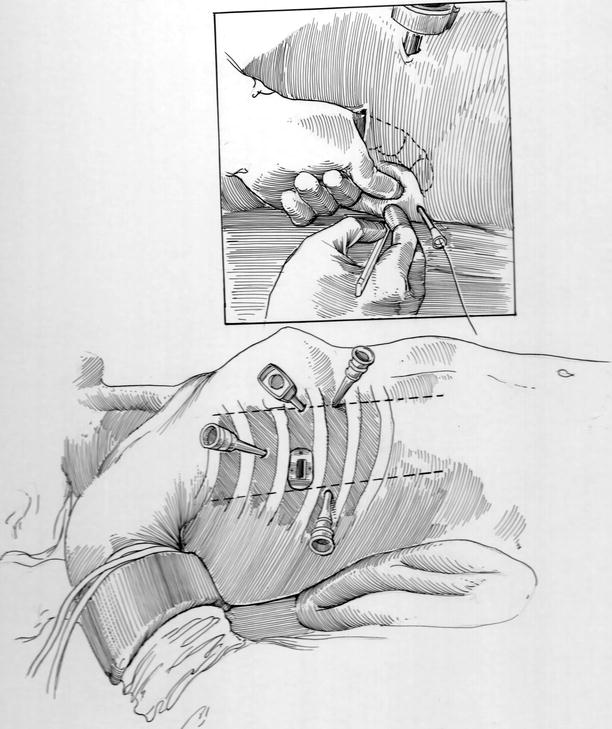
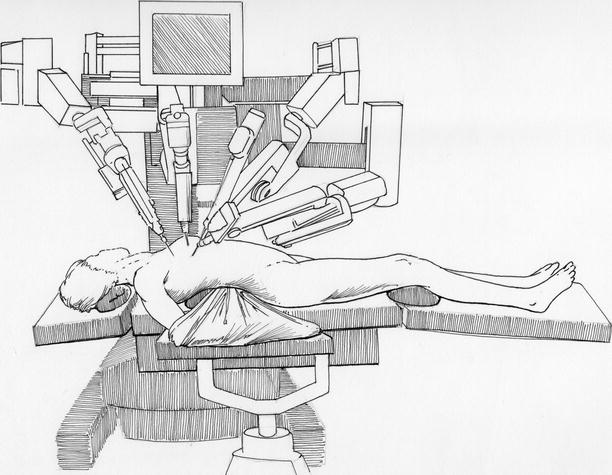

Fig. 23.3
“Safe” trocar insertion and pericardial retraction sutures: the surgeon can direct “safe” trocar placement by placing an index finger through the working port and palpating insertion sites. (Inset) Intercostal space trocars (ICS) are shown for the left instrument arm (3d ICS), the right instrument arm (5th ICS—posterior axillary line), the 3-D camera (4th ICS—anterior), and the dynamic atrial retractor (5 or 6th ICS—anterior axillary line). The inset shows a pericardial retraction suture emerging from a trans-thoracic angio-catheter

Fig. 23.4
Robot instrument cart deployment: for “docking” to instrument and camera trocars, the draped robotic instrument cart is deployed from the left side of the operating table. Thereafter, individual instruments are inserted and attached to the sterile electronic adapter, which conveys instructing digital signals from the surgeon’s operating controls
After establishing cardiopulmonary perfusion the pericardium is opened longitudinally and well anterior to the phrenic nerve. Thereafter, pericardial retraction sutures are placed along the posterior edge of the cut pericardium and brought through the chest wall, using 14—gauge angiocatheters, and then distracted laterally for cardiac exposure (Fig. 23.3—Inset).
Vascular Cannulation and Intraclude™ Balloon Occluder Placement
Many surgeons prefer to use separate internal jugular/superior vena cava (SVC) and femoral vein/right atrial venous drainage cannulas. This assures the best cardiac decompression, which enhances mitral valve visualization and provides optimal myocardial systemic cooling. However, a large single femoral vein to SVC cannula can provide the same level of venous drainage, if positioned correctly and remains un-kinked during left atrial retraction. When used alone, a 22 or 25 Fr Edwards-Quickdraw™ (Edwards Lifesciences, Irvine, CA) femoral vein cannula works well, if advanced into the SVC. Again, it should be placed using the guide-wire technique and directed by trans-esophageal echocardiography (Fig. 23.5).
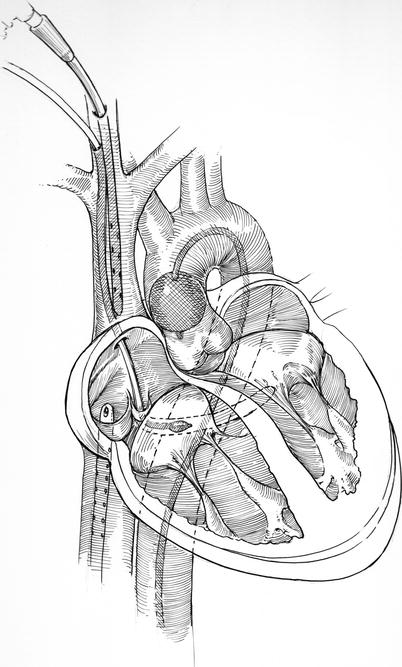

Fig. 23.5
IntraClude™ Technique (Medtronic Inc, St Paul, MN): as described in the text, the original Port Access™ minimally invasive surgical platform, included: the aortic balloon IntraClude™, a large Quickdraw™ venous drainage cannula, a coronary sinus catheter, and a pulmonary artery vent. The IntraClude™ catheter provides a lumen to administer antegrade cardioplegia and vent the aortic root. After inflation the balloon must remain securely positioned above the aortic valve and coronary ostia. If needed the pulmonary vent and coronary sinus catheters are inserted via the right internal jugular vein. Surgeons have tended to omit the use of the pulmonary vent when they can achieve adequate venous drainage
Heartport, Inc. (Mountain View, CA) marketed the intra-aortic balloon EndoClamp™ first in the late 1990s. This earlier version newer IntraClude™ device (Edwards Lifesciences Inc., Irvine, CA) was the first of its kind to be introduced into cardiac surgery. The term Port-Access™ was used initially to describe the overall Heartport™ platform for performing minimally invasive cardiac surgery. In addition to the EndoClamp™ the entire Heartport™ platform included a retrograde coronary sinus cardioplegia catheter that was inserted from the jugular vein and positioned either under echocardiography or angiography. Also, a trans-venous pulmonary artery vent was included with the system to decompress the heart (Fig. 23.5).
Today, the term “thru-port” is applied to describe operations done through small ports rather than large incisions and particularly when endo-aortic balloon catheter occlusion (now updated as the IntraClude™ device) is used. Early series of Port-Access™ mitral valve surgery reported a number of vascular and neurological complications associated with retrograde perfusion and balloon catheter passage into the proximal aorta [2, 3]. However, with the evolution of this technology and more frequent use by several experts, safety improved and the advantages of this technique emerged [4, 5]. Nevertheless, the potential major complications of using the IntraClude™ device still are retrograde aortic dissection, innominate artery origin occlusion, and balloon displacement into the left ventricle, which could render inadequate myocardial protection. At the same time advantages of using intra-aortic balloon occlusion include the absence of a mechanical cross clamp with the need for a direct aortic cardioplegia needle placement, that it can be used in re-operative situations, and that it can be placed prior to going on bypass. In addition, with a competent aortic valve, the catheter can be used to administer antegrade cardioplegia as well as vent air from the aortic root.
When using the intra-aortic balloon occlusion technique, a specialized EndoReturn™ (21 Fr or 23 Fr) (Edwards Lifesciences, Inc., Irvine, CA) femoral arterial cannula is used for perfusion. This device has an occlusive valve for passage of the guide-wire directed balloon catheter into the aorta (Fig. 23.6). This platform necessitates a larger arterial cannula than is used with the trans-thoracic aortic clamp method (see Chaps. 5, 20, and 24). Nevertheless, as the IntraClude™ balloon catheter decreases the cross-sectional area of the arterial cannula, it is wise to monitor perfusion pressures carefully. When the perfusion pressure becomes elevated above 350 Torr, the opposite femoral artery should be cannulated, as an additional inflow site, to decrease the line pressure and limit the risk of a retrograde aortic dissection.
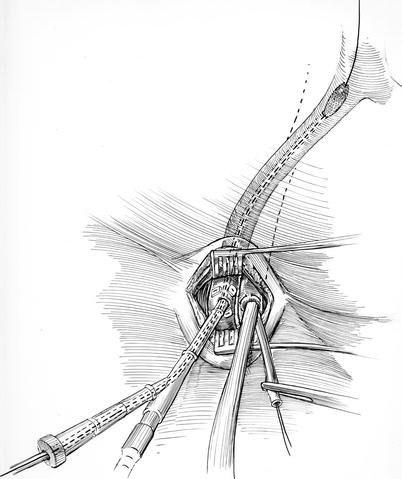

Fig. 23.6
IntraClude™ insertion: here, the EndoReturn™ arterial cannula has been inserted. Over a guide-wire, the IntraClude™ balloon is being advanced through the hemostatic “sidearm valve” toward the ascending aorta under trans-esophageal echocardiographic guidance. If the IntraClude™ balloon reorientation becomes necessary during the operation, the guide-wire can be reinserted, the valve loosened, and the catheter repositioned
Cardiopulmonary Perfusion and Operative Basics
Vacuum-assisted venous drainage is used throughout these operations. The systemic perfusion temperature is allowed to drift. The IntraClude™ balloon should be monitored by TEE throughout the cardiac arrest time to be sure that adequate aortic occlusion persists and that antegrade cardioplegia is being delivered optimally. Carbon dioxide is insufflated continuously into the thorax to displace intra-cardiac air. Perfusion characteristics and the endo-balloon pressure should be monitored closely throughout each operation.
Two retraction sutures are placed along the posterior edge of the opened pericardium and then passed through right lateral chest wall to affect cardiac exposure. After the heart is rendered asystolic briefly by an adenosine injection,(30 mg) the IntraClude™ balloon is inflated and stabilized just above the aortic valve. A large dose of antegrade cold blood cardioplegia is infused through the balloon catheter to establish a continuous asystolic cardiac arrest. Smaller infusions are given every 20–30 min to assure maximal cardiac protection. Using the robotic curved scissors, the left atrium is opened along the inter-atrial groove with care to not injure the pulmonary vein orifices. The mitral apparatus is then exposed with the dynamic retractor [6]. Thereafter, the mitral valve repair is done by the following techniques.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


