The aim of this study was to investigate the association between major adverse cardiovascular events (MACEs) and inducible ischemia on regadenoson cardiac magnetic resonance (CMR) myocardial perfusion imaging (MPI) performed at 3.0 T. Regadenoson stress CMR MPI is increasingly used to assess patients with suspected ischemia; however, its value in patient prognostication and risk reclassification is only emerging. A total of 346 patients with suspected ischemia who were referred for regadenoson CMR were studied. The prognostic association of presence of inducible ischemia by CMR with MACEs was determined. In addition, we assessed the extent of net reclassification improvement by CMR beyond a clinical risk model. There were 52 MACEs during a median follow-up period of 1.9 years. Patients with inducible ischemia were fourfold more likely to experience MACEs (hazard ratio, 4.14, 95% confidence interval 2.37 to 7.24, p <0.0001). In the best overall model, presence of inducible ischemia conferred a 2.6-fold increased hazard for MACEs adjusted to known clinical risk markers (adjusted hazard ratio 2.59, 95% confidence interval 1.30 to 5.18, p = 0.0069). Patients with no inducible ischemia experienced a low rate of cardiac death and myocardial infarction (0.6% per patient-year), whereas those with inducible ischemia had an annual event rate of 3.2%. Net reclassification improvement across risk categories (low <5%, intermediate 5% to 10%, and high >10%) by CMR was 0.29 (95% confidence interval 0.15 to 0.44), and continuous net reclassification improvement was 0.58. In conclusion, in patients with clinical suspicion of myocardial ischemia, regadenoson stress CMR MPI provides robust risk stratification. CMR MPI negative for ischemia was associated with a very low annual rate of hard cardiac events. In addition, CMR MPI provides effective risk reclassification in a substantial proportion of patients.
Regadenoson, an A2A adenosine receptor agonist, has become one of the most commonly used stress agents for myocardial perfusion imaging in the United States since its approval in 2008. The widespread use of regadenoson relates to its longer half-life, which allows a more convenient fixed-dose hand injection rather than the continuous infusion required for adenosine. Pharmacologic vasodilator cardiac magnetic resonance (CMR) myocardial perfusion imaging (MPI) has been shown to have excellent diagnostic utility in numerous single- and multicenter studies as well as the ability to forecast clinical outcomes. The effectiveness of CMR MPI may be further enhanced by the improved contrast-to-noise ratio of 3-T imaging. With recent guidelines from the American Heart Association and American College of Cardiology recommending the use of CMR MPI as a reasonable test for the evaluation of patients with suspected ischemic heart disease, it is expected that the clinical use of regadenoson vasodilator CMR MPI will increase. Although there have been promising pilot data using regadenoson, these studies have involved a small number of patients and have been conducted on 1.5-T systems. In the present study, we tested the hypothesis that regadenoson vasodilator CMR MPI performed at 3 T provides strong prognostic value in patients suspected to have myocardial ischemia.
Methods
We prospectively studied 357 consecutive patients clinically referred for CMR assessment of myocardial ischemia. Patients were included if they were >18 years of age and referred for assessment of symptoms suspicious of coronary artery disease. Exclusion criteria included severe renal dysfunction (glomerular filtration rate <30 ml/min), acute coronary syndromes, pregnancy, or absolute contraindication to magnetic resonance imaging. A detailed medical history was obtained before each examination.
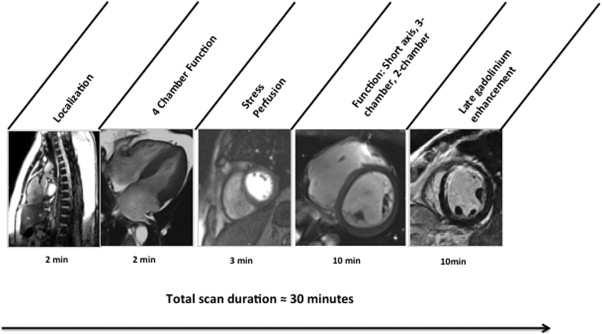
We performed all regadenoson CMR MPI on a 3.0-T scanner with a 16-element coil (Tim Trio/Verio; Siemens Healthcare, Erlangen, Germany) using a protocol consisting of vasodilator myocardial perfusion, ventricular function, and late gadolinium enhancement (LGE) imaging ( Figure 1 ). All images were acquired with vector electrocardiographic gating during breath-hold. Cine steady-state free precession (typical repetition time 3.4 ms, echo time 1.2 ms; in-plane spatial resolution 1.6 × 2.0 mm) was used for imaging left ventricular (LV) size and function. Myocardial perfusion images were acquired at 3 short-axis segments (basal, midventricular, and apical) and 4-chamber long-axis orientation during bolus injection of 0.1 mmol/kg intravenous gadolinium diethylenetriamine penta-acetic acid (Magnevist; Bayer, Wayne, New Jersey) for stress imaging. A saturation-recovery prepared turbo fast low-angle single-shot gradient-echo sequence (typical repetition time 2.4 ms, typical echo time 1.0 ms, typical flip angle 18°, 10-ms delay after saturation before readout, linear phase-encoding order, acceleration factor 2) with the use of generalized auto-calibrating partial parallel acquisition (in-plane resolution 2.2 × 2.7 mm, slice thickness 10 mm, receiver bandwidth 800 to 900 Hz per pixel). Regadenoson (Astellas Pharma US, Deerfield, Illinois) was used as the stress agent in all studies. LGE imaging was performed at 10 to 15 minutes after contrast.
All images were analyzed with commercial software (QMASS; Medis Medical Imaging, Leiden, The Netherlands) at the consensus of 2 independent readers blinded to clinical data. LV volumes (indexed to body surface area) and the LV ejection fraction were obtained by manual tracing of end-diastole and end-systole. LGE was semiautomatically quantified using full-width half-maximum methods and calculated as total infarct mass and percentage of total myocardial mass. A stress perfusion defect was defined as a hypoenhanced region >1 pixel in thickness that persisted for ≥3 phases after peak contrast enhancement in a coronary distribution. Presence of ischemia was defined as presence of a stress perfusion defect in any segment without corresponding LGE. Extent of ischemia was designated on the basis of the number of segments out of the American Heart Association and American College of Cardiology 17-segment model.
Clinical follow-up after CMR was obtained by mailed questionnaire, review of medical records, and contact with patients’ cardiologists. Patients were contacted by telephone if the mailed questionnaire was not returned, and a standardized set of questions was used. We used a composite end point of major adverse cardiovascular events (MACEs) that included cardiac death, new myocardial infarction (MI), late coronary revascularization (>90 days after CMR MPI), ventricular arrhythmia (ventricular fibrillation or sustained ventricular tachycardia), and hospitalization for unstable angina or heart failure. We also assessed the association of CMR MPI findings with a hard composite outcome of cardiac death or acute MI. Cardiac death was defined as death preceded by MI, ventricular arrhythmia, hospitalization for heart failure, or unstable angina. Ventricular arrhythmias were confirmed on telemetry or interrogation of pacemakers or defibrillators, where available. The Social Security Death Index was used to confirm all cases of death. Time to event was calculated as the period between CMR MPI study and the first occurrence of a MACE. Patients who did not experience MACEs were censored at noncardiac death or last follow-up.
Continuous and categorical variables were compared by Student’s t test or Wilcoxon’s rank-sum test (depending on data normality) and Fisher’s exact test, respectively. Event-free survival for those with inducible ischemia was analyzed by Kaplan-Meier estimates (using a log-rank test). Univariate associations between clinical and CMR covariates with MACEs were assessed by Cox proportional-hazards regression modeling. We built a multivariate clinical risk model with a backward elimination Cox regression strategy using p <0.05 as the criterion to remain in the model. We also performed logistic regression analyses to determine the prognostic association of inducible ischemia presence with MACEs within the initial 3 years after CMR study. Finally, we assessed whether inducible ischemia by CMR MPI led to net reclassification improvement (NRI) of patient risk, using a validated method. A 2-sided p-value <0.05 was considered statistically significant. All statistical analysis was performed with SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
Results
We studied 357 consecutive patients referred for CMR MPI. Presenting symptoms included chest pain (n = 162 [45%]), new-onset cardiomyopathy (n = 91 [25%]), dyspnea (n = 69 [19%]), abnormal electrocardiographic findings (n = 24 [7%]), and syncope (n = 11). Eleven patients (3%) were excluded for technical reasons. Baseline characteristics stratified by inducible ischemia are listed in Table 1 . Ninety-three patients (27%) demonstrated inducible ischemia. They were more likely to be older, to be male, to have high prevalence of coronary risk factors, and to have lower LV ejection fractions (47% vs 56%, p <0.0001).
| Variable | All Patients (n = 346) | Inducible Ischemia | p-Value (Inducible Ischemia vs. No Inducible Ischemia) | |
|---|---|---|---|---|
| No (n = 253) | Yes (n = 93) | |||
| Age (years) | 55.1 ± 14.8 | 52.6 ± 14.3 | 61.9 ± 13.8 | <0.0001 |
| Women | 136 (39.3%) | 110 (43%) | 26 (28%) | <0.01 |
| Body mass index (kg/m 2 ) | 28.4 ± 6.6 | 28.1 ± 6.9 | 29.1 ± 4.8 | 0.23 |
| Resting Systolic Blood Pressure (mmHg) | 127.2 ± 18.6 | 127.9 ± 18.5 | 125.3 ± 18.8 | 0.25 |
| Resting Diastolic Blood Pressure (mmHg) | 70.0 ± 12.8 | 70.1 ± 12.5 | 69.7 ± 13.6 | 0.78 |
| Hypertension | 173 (51%) | 118 (47%) | 55 (61%) | <0.05 |
| Diabetes | 54 (16%) | 31 (12%) | 23 (25%) | <0.01 |
| Smoker | 55 (16%) | 32 (13%) | 23 (26%) | <0.01 |
| Hypercholesterolemia | 140 (41%) | 82 (32%) | 58 (65%) | <0.0001 |
| Aspirin use | 161 (47%) | 94 (37%) | 67 (74%) | <0.0001 |
| Beta-blocker use | 173 (50%) | 107 (42%) | 66 (73%) | <0.0001 |
| ACE inhibitor/ARB use | 159 (46%) | 114 (45%) | 45 (49%) | 0.54 |
| Statin use | 27 (8%) | 15 (6%) | 12 (13%) | <0.05 |
| Nitrate use | 39 (11%) | 15 (6%) | 24 (26%) | <0.0001 |
| Calcium Channel Blocker use | 42 (12%) | 35 (14%) | 7 (8%) | 0.14 |
| Left ventricular ejection fraction (percent) | 53.9 ± 14.8 | 56.4 ± 15.8 | 47.4 ± 18.1 | <0.0001 |
| Left ventricular end diastolic volume index (mL/m 2 ) | 93.4 ± 16.9 | 88.3 ± 32.8 | 106.9 ± 44.4 | <0.0001 |
| Left ventricular end systolic volume index (mL/m 2 ) | 47.4 ± 38 | 42.0 ± 33.0 | 61.9 ± 46.1 | <0.0001 |
| Left ventricular mass (grams) | 118.4 ± 49.7 | 111.0 ± 47.5 | 136.7 ± 50.7 | <0.0001 |
| Right ventricular ejection fraction (percent) | 53.8 ± 9.63 | 54.4 ± 9.4 | 52.3 ± 10.0 | 0.10 |
| Right ventricular end diastolic volume index (mL/m 2 ) | 73.1 ± 21.3 | 72.8 ± 20.4 | 73.9 ± 23.6 | 0.69 |
| Right ventricular end systolic volume index (mL/m 2 ) | 34.6 ± 15.5 | 33.9 ± 14.2 | 36.4 ± 18.6 | 0.19 |
| Presence of late gadolinium enhancement | 145 (42%) | 77 (30%) | 68 (73%) | <0.0001 |
| Late gadolinium enhancement mass (grams) | 5.43 ± 12.21 | 3.7 ± 11.2 | 10.6 ± 13.7 | <0.0001 |
The remaining 346 patients were followed for a median of 1.9 years (interquartile range 1.3 years). There were 52 MACEs (4 cardiac deaths, 4 acute MIs, 6 unstable angina hospitalizations, 26 heart failure hospitalizations, 7 arrhythmias, and 5 late coronary revascularizations) during the entire follow-up period. In the initial 3 years after CMR MPI scanning, 45 of the 52 MACEs occurred. No major complications occurred because of regadenoson administration.
In Kaplan-Meier analysis of MACE-free survival, patients with inducible ischemia experienced worse MACE-free survival compared with those without inducible ischemia (p <0.001; Figure 2 ). Cumulative MACE rates stratified by the presence of inducible ischemia were shown in Figure 3 . By univariate Cox regression, patients with inducible ischemia were fourfold more likely to experience MACEs (hazard ratio 4.14, p <0.0001; Table 2 ). For every segment of myocardial ischemia, hazards to MACEs on average increased by 12% (hazard ratio 1.12, p = 0.0029). Patient age, history of coronary bypass surgery, LV end-diastolic volume index, and LV end-systolic volume index were selected to form the clinical risk model for MACEs. When the presence of inducible ischemia by CMR MPI was added to this clinical model, it substantially improved the model (Likelihood ratio chi-square increased from 37.25 to 44.41, p <0.01; Table 3 ). Adjusted for the effects of the clinical risk model, patients with inducible ischemia were 2.6-fold more likely to experience MACEs (adjusted hazard ratio 2.59, 95% confidence interval 1.30 to 5.18, p = 0.0069). Annualized MACE rates were 5.2% (per patient-year) in patients without inducible ischemia and 17.5% in patients with ischemia ( Figure 4 ).
| Univariable Associations | |||
|---|---|---|---|
| Characteristic | Hazard Ratio (95% CI) | Likelihood Ratio χ 2 | p-Value |
| Demographics | |||
| Age | 1.03 (1.02–1.06) | 11.1 | 0.0009 |
| Women | 0.66 (0.36–1.21) | 1.81 | 0.18 |
| Body Mass Index, per kg/m 2 | 1.00 (0.96–1.04) | 0.0002 | 0.99 |
| Hypertension | 1.78 (0.99–3.22) | 3.73 | 0.05 |
| Diabetes Mellitus | 1.88 (0.99–3.53) | 3.80 | 0.05 |
| Smoker | 1.89 (0.99–3.56) | 3.83 | 0.05 |
| Dyslipidemia | 1.63 (0.92–2.86) | 2.85 | 0.09 |
| Coronary artery bypass grafting | 4.39 (1.97–9.78) | 13.0 | 0.0003 |
| Myocardial infarction | 3.79 (2.06–7.01) | 18.2 | <0.0001 |
| Percutaneous coronary intervention | 2.69 (1.43–5.08) | 9.38 | 0.002 |
| Medications | |||
| Aspirin use | 3.29 (1.77–6.11) | 14.1 | 0.0002 |
| Beta-blocker use | 1.42 (0.80–2.51) | 1.45 | 0.23 |
| ACE-inhibitor use | 1.89 (1.05–3.37) | 4.59 | 0.03 |
| Statin use | 2.10 (1.18–3.75) | 6.34 | 0.01 |
| Calcium channel blocker use | 2.13 (1.06–4.29) | 4.50 | 0.03 |
| Electrocardiographic findings | |||
| Long QT interval (corrected) | 4.53 (2.30–8.91) | 19.1 | <0.0001 |
| Left bundle branch block | 1.28 (0.51–3.23) | 0.27 | 0.60 |
| Right bundle branch block | 2.70 (1.07–6.85) | 4.39 | 0.04 |
| Left ventricular hypertrophy | 1.41 (0.60–3.31) | 0.62 | 0.43 |
| Q-wave | 3.65 (1.99–6.56) | 17.9 | <0.0001 |
| CMR findings | |||
| Left ventricular ejection fraction | 0.95 (0.94–0.97) | 34.9 | <0.0001 |
| Left ventricular end diastolic volume index | 1.01 (1.00–1.02) | 23.3 | <0.0001 |
| Left ventricular end systolic volume index | 1.01 (1.01–1.02) | 32.1 | <0.0001 |
| Presence of late gadolinium enhancement | 4.88 (2.54–9.33) | 22.8 | <0.0001 |
| Inducible Ischemia | 4.14 (2.37–7.24) | 24.7 | <0.0001 |
| Ischemia Severity | 1.12 (1.04–1.20) | 8.89 | 0.0029 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


