Risk Stratification and Post–Myocardial Infarction Therapy
Unstable angina, non–ST-segment-elevation myocardial infarction (NSTEMI), and ST-segment-elevation myocardial infarction (STEMI) represent a spectrum of ischemic coronary disease of similar etiology—atherosclerotic plaque instability and rupture—termed acute coronary syndrome (ACS). The role of risk stratification in ACS is to identify which patients have increased risk for adverse events and are therefore more likely to benefit from the array of mechanical and pharmacologic therapies available. Risk stratification after myocardial infarction (MI) begins during the initial clinical encounter, continues throughout the index hospitalization, and remains important after discharge. Although modern advances have had a significant impact on outcomes of MI, post-ACS morbidity (and mortality) remain a challenging problem, most notably recurrent ischemia and infarction, congestive heart failure (CHF), and sudden cardiac death (SCD).
Recent data favor an early invasive approach for most patients. Early aggressive lipid-lowering therapy post-MI is clearly beneficial, with lower targets for low-density lipo-protein (LDL) levels. The importance of neurohormonal blockade has become apparent, particularly in patients with left-ventricular systolic dysfunction. Antiplatelet therapies have become essential, both during and after ACS, but in particular following percutaneous coronary intervention (PCI). The role of the implantable cardioverter–defibrillator (ICD) after MI has become clearer, with recent trials indicating no benefit to ICD early in the course post-MI, but demonstrable subsequent benefit in patients with significant left ventricular (LV) dysfunction. The recognition of the role of inflammation in ACS has led to the development of clinical assays measuring inflammatory markers such as C-reactive protein (CRP) and brain natriuretic peptide (BNP), but their role in clinical practice is not well established. Lifestyle modification and risk-factor reduction remain important, including smoking cessation, diabetes, and hypertension management.
RISK STRATIFICATION FOR ST-ELEVATION MI
Identification of high-risk characteristics early after MI is important because 25% of deaths during the first postinfarction year occur within the first 48 hours of hospitalization, and more than one-half of deaths occur within the first month after STEMI. Demographic and clinical data, electrocardiogram (ECG), serum markers, and various diagnostic tests assist in the risk assessment.
Initial Presentation
Clinical and Demographic Factors
The most important predictors of death within 30 days are age, systolic blood pressure (SBP) and heart rate at presentation, evidence of CHF on physical examination, location of infarction, and previous infarction. In the Global Utilization of Streptokinase and TPA for Occluded Arteries-I (GUSTO-1) study, these predictors accounted for >90% of the total prognostic information. Additional important prognostic factors include female gender, history of diabetes, hypertension, smoking, and vascular disease (Table 43.1).
TABLE
43.1 Predictors of Mortality in STEMI
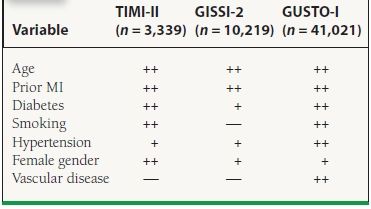
+ , univariate predictor; ++, multivariate predictor.
Advanced age has been recognized as an important predictor of mortality in several studies. In the NRMI (National Registry of Myocardial Infarction) registry, a community-based database with information on >350,000 patients with acute MI at U.S. hospitals, in-hospital mortality ranged from 3% for patients younger than 55 years of age to 28% for individuals more than 84 years of age. Older patients are more likely to possess a history of a prior MI, have more severe coronary disease, and consequently are more likely to develop CHF and cardiogenic shock after MI. Additionally, several reports have shown that older patients are also less likely to receive life-saving therapies such as immediate reperfusion therapy, beta-blockers, and aspirin, which may contribute to the worsened prognosis.
In several studies, women have been shown to have higher mortality after STEMI. In the GUSTO-1 trial, women had higher 30-day mortality (11.3% vs. 5.5%), occurrence of shock (9% vs. 5%), and reinfarction (5.1% vs. 3.6%) compared to men. Part of this increased risk can be explained by the advanced age and increased prevalence of preexisting diabetes and hypertension. Additionally, women are more likely to present late during an infarction.
Paradoxically, smokers possess a lower risk for early mortality, most likely because of their younger age. Diabetes mellitus has been associated with a 1.5 to 3.0 times higher mortality after STEMI. Whether this is due to a higher atherosclerotic burden or some other characteristic induced by the diabetic state, such as silent ischemia or a larger infarct size, remains unclear. Further, the nonfatal complications are also higher in diabetic patients, including a greater incidence of postinfarction angina, reinfarction, and heart failure.
Physical Examination
The clues to right ventricular (RV) and LV dysfunction on physical examination provide the most important prognostic information. Accordingly, variables predictive of a worsened outcome include hypotension, tachycardia, jugular venous distension, an S3 gallop, pulmonary edema, and evidence of peripheral hypoperfusion, many of which are captured by the Killip classification (Table 43.2). The physical examination can also help to identify mechanical complications of MI, such as acute mitral regurgitation, ventricular septal defect, and free wall rupture, all of which have been associated with significant mortality.
TABLE
43.2 Killip Classification and Mortality from GUSTO-I Trial
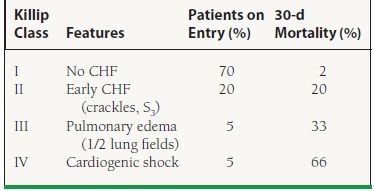
CHF, congestive heart failure.
Electrocardiogram
The ECG provides useful information about the location and size of infarction, likelihood of tissue reperfusion after treatment, presence of ongoing ischemia, and conduction system dysfunction. The finding of ST elevation or depression has similar prognostic implications (Fig. 43.1). Mortality is greater in patients experiencing anterior wall MI compared to after inferior MI, even when corrected for infarct size. Patients with RV infarction complicating inferior infarction have a higher mortality rate than patients sustaining an inferior infarction without RV involvement. Patients with multiple leads showing ST-segment elevation and those with a high degree of ST-segment elevation have increased mortality, especially if their infarct is anterior. Patients with persistent or advanced heart block (e.g., Mobitz type II, second-degree, or third-degree AV block) or new intraventricular conduction abnormalities (bifascicular or trifascicular) in the course of an acute MI have a worse prognosis than do patients without these abnormalities. The influence of high-grade conduction block is particularly important in patients with RV infarction, for such patients have a markedly increased mortality. Other ECG findings suggesting a worse outcome are persistent horizontal or downsloping ST-segment depression, Q waves in multiple leads, evidence of RV infarction accompanying an inferior infarction, ST-segment depressions in anterior leads in patients with an inferior infarction, and atrial arrhythmias (especially atrial fibrillation).
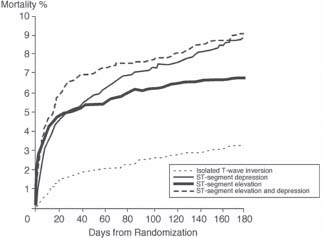
FIGURE 43.1 Mortality rate according to electrocardiographic findings on presentation with acute MI in the GUSTO IIb trial. (Adapted from Savonitto S, Ardissino D, Granger CB, et al. Prognostic Value of the Admission Electrocardiogram in Acute Coronary Syndromes.JAMA 1999;281:707–713.)
Other than these well-established predictors on ECG, ST-segment resolution has generated renewed interest in determining effectiveness of reperfusion therapy. Resolution of ST elevation predicts successful perfusion at the myocardial level, which is the most important predictor of LV function and survival. Continuous ST-segment monitoring has been shown to yield important prognostic information after 60 minutes of observation. In the ASSENT 2 (Assessment of Safety and Efficacy of a New Thrombolytic) and ASSENT-PLUS studies, the optimal cutoff for ST-segment resolution analyses was found to be 50%, measured at 60 minutes. Patients with ST resolution (40%) by this criterion had a 30-day mortality of only 1.4%.
Biomarker Assessment
Two separate groups of biomarkers have been used to predict outcome after MI. One group includes the myocardial enzymes that predict infarct size and another group assesses the degree of systemic vascular inflammation. The prognostic value of CRP, endothelin, BNP, CD40, and CD40 ligand has recently been investigated extensively. These markers of inflammation seem to predict an active atherosclerotic disease process. Aggressive risk-factor modification may be more important when the levels of these markers are high.
More conventional markers of myocardial damage include troponin-I or -T, creatine kinase (CK), CK-MB 1 and 2 isoforms, CK-MB isoenzyme mass, and occasionally myoglobin. The presence and degree of troponin, CK, and CK-MB isoenzyme elevation on admission and thereafter have been associated with poorer outcome in the setting of both STEMI and NSTEMI. However, there is less information on troponin levels in STEMI. In the GUSTO IIa study, 30-day mortality was substantially higher among patients who were troponin-T positive. Given their more rapid return to baseline, CK and CK-MB isoenzymes are also helpful for identifying high-risk individuals by facilitating the diagnosis of reinfarction shortly after an STEMI or NSTEMI. Currently, infarct size is determined by CK-MB mass; the role of troponin-I or -T in this matter has been less well established.
Imaging
Imaging at the time of acute infarction is used to determine the amount of jeopardized myocardium. Contrast echocardiography and technetium-based imaging can be used to quantify perfusion noninvasively. Nuclear scanning is superior for quantifying perfusion, whereas echocardiography is better for assessing function. Acute imaging has been used principally in clinical trials to determine the degree of myocardial salvage, which is the percent of ischemic myocardium atpresentation that has adequate perfusion on follow-up.
During Hospitalization
Recurrent angina is an important predictor of a worsened outcome and the need for revascularization. Recurrent chest pain frequently signifies ischemic myocardium, either in the peri-infarct territory supplied by the infarct-related artery or ischemia at a distance secondary to a non–infarct-related artery. Early revascularization is required in many patients who have postinfarct angina. Other important predictors include LV or RV dysfunction and mechanical complications of MI. Cardiogenic shock possesses a very high mortality in which medical management is not effective. Early revascularization in patients who develop cardiogenic shock within 36 hours of an MI is recommended, based on the findings of the SHOCK (Should We Emergently Revascularize Occluded Coronary Arteries for Cardiogenic Shock) trial, which showed reduced mortality with early revascularization compared to medical stabilization (33.3% vs. 51.6%). Arrhythmias, including high-grade AV block, atrial fibrillation, or ventricular tachycardia, also predict poor outcome.
Predischarge Assessment
Although significant emphasis is placed on predischarge risk stratification, many high-risk patients will declare themselves clinically during their hospital stay. The challenge for the clinician during the predischarge phase is to distinguish the few patients who remain at higher risk from the many relatively lower-risk patients. Although multiple testing technologies have been developed to aid in this process, the low event rate in these patients (1-year mortality rates of 2% to 5%) mandates that these tests must be highly sensitive and specific if they are to have clinical value. What tests should be routinely performed for predischarge risk stratification is highly debated. Risk stratification at discharge can be accomplished by determining three factors: (a) resting LV function, (b) residual potentially ischemic myocardium, and (c) susceptibility to serious ventricular arrhythmias. More sophisticated testing may provide additional data but may not be as useful in changing patient outcomes.
LV Function Assessment
Assessment of LV function is typically performed by echocardiography or by ventriculography at the time of cardiac catheterization. However, imaging of the left ventricle at rest may not distinguish between infarcted, irreversibly damaged myocardium and hibernating myocardium. Therefore, many different techniques have been used to determine viable myocardium, including dobutamine echocardiography, rest-redistribution thallium, positron emission tomography (PET) scanning, and magnetic resonance imaging (MRI). Dobutamine echocardiography can provide functional assessment along with information on viability and ischemia. However, the results are directly dependent on the expertise and experience of the interpreter. Radionucleotide imaging provides higher sensitivity to detect ischemia, but specificity can be compromised by the size of the patient, diaphragmatic or breast attenuation. Further, regional wall motion assessment is not as precise as with echocardiography. Regardless of the imaging modality chosen, the prognosis is worse if there is significant LV dysfunction, or if there is a large amount of ischemic myocardium.
Stress Testing
Patients who do not have high-risk features after successful thrombolysis should be considered for exercise stress testing. Although the predictive accuracy of exercise stress testing has diminished in the reperfusion era as a result of the lower incidence of adverse outcomes, it is still given a Class I indication under current American College of Cardiology/American Heart Association (ACC/AHA) guidelines. In addition, although it is not known whether exercise testing can effectively risk-stratify patients who have not received acute reperfusion therapy, it is also assumed to be effective in this setting. Low-level exercise appears to be safe in patients who have been free of angina or heart failure and who possess a stable baseline ECG during the previous 2 to 3 days. Patients who are unable to exercise or who have baseline ECG abnormalities that would preclude interpretation should undergo an exercise test with imaging. Patients who cannot achieve a 3 or 4 MET workload, those who develop ischemia at a low level of exercise, or those in whom blood pressure (BP) drops during exercise should undergo coronary angiography. No further testing should be necessary in patients without these high-risk findings.
Assessment for Risk of Sudden Cardiac Death
Determination of risk for SCD after MI is important because it is highest in the first 1 to 2 years after the index event. The most important predictor for SCD is LV dysfunction. Provocative electrophysiology studies are not necessary for risk stratification. Signal-averaged ECG, heart-rate variability, QT dispersion, and baroreflex sensitivity have been investigated to select specific patients with LV dysfunction who might benefit from an ICD. The presence of a filtered QRS complex duration >120 milliseconds and abnormal late potentials recorded on a signal-averaged ECG after acute MI signifies somewhat higher risk for SCD. However, the signal-averaged ECG suffers from a high false-positive rate, which makes the test clinically less useful. Depressed heart-rate variability is an independent predictor of mortality and arrhythmic complications after acute MI. A depressed baroreflex sensitivity value (3.0 millisecond/mm Hg) is associated with about a threefold increase in the risk of mortality. These tests may provide useful prognostic information, but at present, only assessment of ejection fraction (EF) is necessary to determine eligibility for a device, where significant LV dysfunction qualifies a patient for ICD placement. Recent data indicate, however, that ICD therapy is not beneficial in the early post-MI period, and should be delayed for at least 1 month after an infarction. ICD implantation is generally deferred for 3 months after revascularization, either surgically or percutaneously, at which time reevaluation of LV function can be performed.
Predischarge Management
In contemporary practice, most patients with MI will undergo cardiac catheterization, even after receiving thrombolytics for STEMI, based on the CARESS in AMI and TRANSFER AMI trials and the most recent guidelines. In the minority of patients who do not undergo catheterization initially, a judgment is made as to the presence of clinical variables indicative of high risk for future cardiac events. Patients with spontaneous episodes of ischemia or depressed LV function who are considered suitable candidates for revascularization based on their overall medical condition should be referred for cardiac catheterization. These patients are at increased risk of recurrent infarction (and subsequent increased mortality), and may benefit from revascularization if severe coronary artery disease (CAD) is identified at catheterization.
NON–ST-ELEVATION ACS
Many patients with ACS present without ST elevation on ECG. It is important to note that although the risk of mortality during the index hospitalization is less than in those with ST-elevation ACS, the prognosis at 1 year is similar (see Fig. 43.1). Typically, the underlying pathophysiology is a high-grade stenosis with plaque rupture, but unlike STEMI, the vessel is not totally occluded. Indeed, fibrinolysis has been shown to be of no benefit and may actually be harmful in this patient cohort. Multiple trials have investigated the role of early angiography and PCI versus conservative management in these patients, and it appears that early invasive strategy in the high-risk population provides the best outcome and may even be more cost effective than a conservative strategy.
Non–ST-Elevation MI Risk Stratification
Initial Presentation
A number of historical features predictive of a worse prognosis following non–ST-elevation ACS have been derived from existing trial data. These features are summarized in Table 43.3. The most important are older age, greater number of cardiac risk factors, known CAD, peripheral vascular or cerebrovascular disease, prior MI, previous PCI or coronary artery bypass graft surgery, history of CHF, a more severe anginal pattern, and the use of aspirin within a week of presentation.
TABLE
43.3 Predictors of a Worse Prognosis in NSTEMI
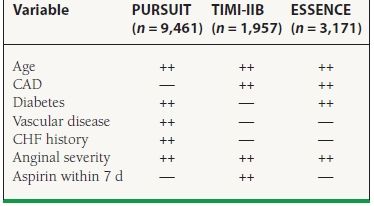
Other study predictors include female gender, number of risk factors, previous MI, prior PCI/CABG.
Electrocardiogram
Among patients with non–ST-elevation ACS, the presence of Q waves, ST changes associated with angina or at presentation (in particular, ST-segment depression), T-wave inversions of significant amplitude (i.e., >0.2 mV), or the absence of ECG changes during angina are important predictors of future events (see Fig. 43.1). When clinical variables are also considered, heart rate and the presence of ST depression on admission ECG are the most important multivariable predictors.
Biomarkers
The presence and degree of troponin elevation on admission and thereafter can identify patients who are at increased risk of experiencing adverse outcomes. Cardiac troponin-I and troponin-T are particularly useful in identifying high-risk patients with non–ST-elevation ACS. Other markers of inflammation, such as CRP, CD-40, CD-40 ligand, fibrinogen levels, or brain natriuretic peptide (BNP) can add to the prognostic information in ACS. Adding multiple markers to assess a patient may add important prognostic information, as illustrated in the OPUS-TIMI 16 (Oral Glycoprotein IIb/IIIa Inhibition with Orbofiban in Patients with Unstable Coronary Syndromes) trial and the TACTICS-TIMI 18 (Treat Angina with Aggrastat and Determine Cost of Therapy with an Invasive or Conservative Strategy) analyses (Fig. 43.2). However, at the present time, there is no clear consensus on how to incorporate these markers in patient management.
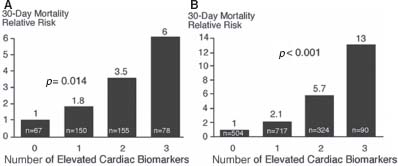
FIGURE 43.2 Relative 30-day mortality risks in OPUS-TIMI 16 (A) and TACTICS-TIMI 18 (B) in patients stratified by the number of elevated cardiac biomarkers (TnI, CRP, and BNP). (From Sabatine MS, Morrow DA, de Lemos JA, et al. Multimarker Approach to Risk Stratification in Non-ST Elevation Acute Coronary Syndromes. Circulation 2002;105:1760, with permission from Wolters Kluwer Health.)



