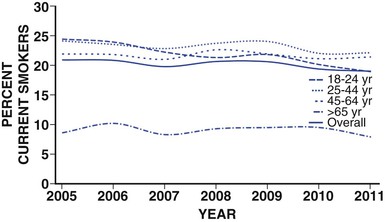
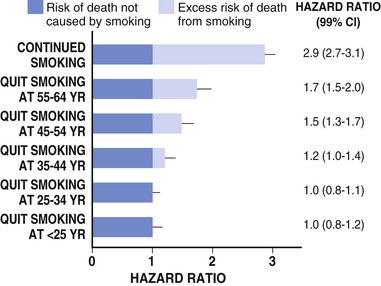
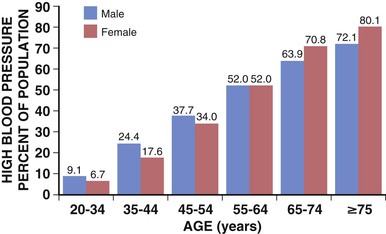
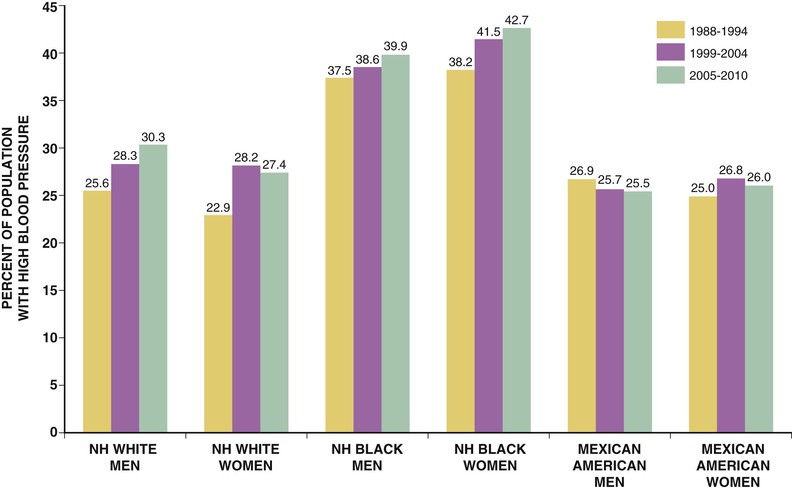
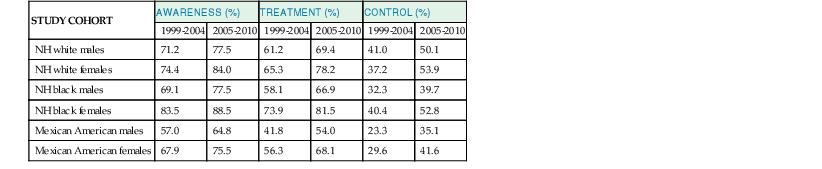
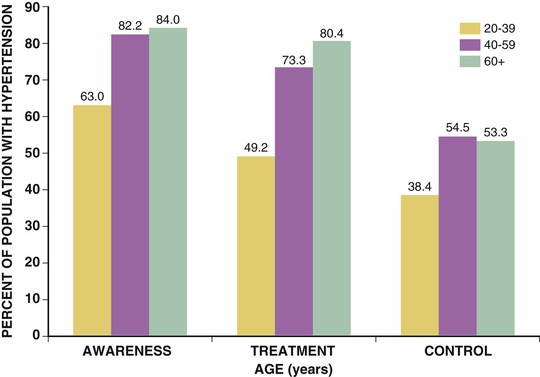
Paul M Ridker, Peter Libby, Julie E. Buring For almost half a century, interventions to reduce the risk of heart attack and stroke among persons without known heart disease have been implemented largely using a two-step process based on absolute risk. First, using a global risk–estimating algorithm such as the Framingham risk score, the Reynolds risk score, or the European Systematic Coronary Risk Evaluation (SCORE),1 physicians have stratified patients who are candidates for primary prevention into lower-, intermediate-, and higher-risk subgroups, typically calculated over a 10-year time frame. Then, guidelines based on such stratification have traditionally targeted lifestyle interventions to those persons at “lower” and “intermediate” risk while limiting more aggressive pharmacologic interventions (such as statin therapy) to those with “higher” risk profiles. Until recently, it was assumed that such a risk-based triage system would distribute primary prevention services efficiently. After all, if the relative benefit of a preventive intervention is similar across all levels of risk, then the greatest absolute benefit will occur among persons with the highest absolute risk. Furthermore, treatment allocation on the basis of high global risk should maximize the benefits of intervention (by targeting those at greatest need) while reducing potential adverse actions and cost (by avoiding exposure to treatment among those with the least need). Currently, however, some in the preventive cardiology community have challenged these long-held beliefs, and have proposed instead that preventive services should be allocated on the basis of proven randomized trial data—that is, “what works?” and “in whom?”—rather than on the basis of an arbitrary scaling of global risk.2 This reconsideration has major implications for how we think about preventive cardiovascular care as well as for guidelines, for the design of future clinical trials, and for emerging preventive concepts such as the “polypill” and broad use of effective generic drugs by prescription or over the counter, independent of individual risk assessment. Consider the situation for statin therapy. Ten years ago, the volume of trial data on the efficacy of hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase inhibitors as an adjunct to diet, exercise, and smoking cessation in specific patient groups was limited, safety data were uncertain, and the cost of treatment was relatively high, particularly for higher-potency statin agents. Thus, facing uncertainty, those writing older guidelines chose appropriately to model the potential benefits of lipid-lowering treatment on the basis of epidemiologic risk scales, even though those scores had never themselves undergone randomized evaluation for improvement of outcomes, nor were they used as trial enrollment criteria. Unfortunately, this system of drug allocation based on epidemiologic modeling rather than completed trials has substantive limitations. First, smoking and hypertension often drive high estimates of global risk, yet the interventions of choice should be smoking cessation and blood pressure reduction rather than reflexive prescription of lipid-lowering therapy. Second, risk prediction models often have proved inadequate in terms of discrimination and calibration in specific patient groups such as ethnic minorities and women. Third, on a population basis, the vast majority of future vascular events occur in persons with intermediate or low 10-year risk estimates, so limiting intervention only to those with highest absolute risk misses large opportunities for prevention. Concepts of lifetime risk suggest that those patients with low 10-year risks often are among those with the highest long-term event rates, for whom early interventions could prove most effective.3 For these and other reasons, many clinicians do not use global risk algorithms routinely. Most problematic, however, results of multiple randomized trials completed since 2005 do not support the notion that statin therapy has constant relative benefits across all risk groups, yet this assumption remains the fundamental justification for arguments to base therapy on absolute risk. Consider the CORONA (Controlled Rosuvastatin Multinational Trial in Heart Failure), AURORA (A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events), 4D (German Diabetes and Dialysis Study), and GISSI-HF (Gruppo Italiano per lo Studio della Sopravvivenza nell’Insufficienza Cardiaca–Heart Failure) trials, which in total included 13,613 patients and were reported between 2005 and 2009.4–7 All four of these well-conducted trials enrolled high-absolute-risk patients who achieved large low-density lipoprotein (LDL) cholesterol reductions with statin therapy. Yet none showed significant clinical benefit. Consider further the JUPITER (Justification for the Use of Statin in Prevention: An Intervention Trial Evaluating Rosuvastatin), AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention Study), and MEGA (Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese) trials that included 32,621 primary prevention patients and were published between 1998 and 2008.8–10 These three trials enrolled low-absolute-risk patients, most of whom would not qualify for statin therapy under any current guideline issued in the United States or Europe. Yet each showed marked benefit of statin therapy. Indeed, these three trials demonstrated the greatest relative risk reductions ever achieved with statin therapy. Taken together, these seven trials present a major challenge to the simplistic idea that absolute risk alone can direct clinically effective allocation of statin therapy. Why then continue to recommend that statins be prescribed on the basis of an epidemiologic calculation of absolute risk? Why not allocate statins instead to patient subgroups proven in clinical trials to benefit from them? A principled, evidence-based interpretation of these recent trials would be not to use statins among patients with renal failure (4D, AURORA) or heart failure (CORONA, GISSI-HF), but to use statins aggressively in primary prevention among those with elevated LDL cholesterol (MEGA), low high-density lipoprotein (HDL) cholesterol (AFCAPS/TexCAPS), or elevated high-sensitivity C-reactive protein (hsCRP) (JUPITER). As just described, few if any of the basic justifications for a “risk-based” approach to statin therapy remain relevant at present. Data on safety now abound, and the evidence base has established that benefits of statin therapy on myocardial infarction (MI), stroke, revascularization procedures, and cardiovascular death outweigh the risks even for those at the lower end of the absolute vascular risk spectrum. This conclusion remains valid even after the modest but statistically significant hazard for diabetes associated with statin use in benefit-to-risk calculations is taken into account.11 Second, almost all statin agents are now off patent, and the cost of treatment has declined dramatically. Third, the cardiovascular community currently has abundant data from many large-scale, randomized, placebo-controlled trials that cover a wide range of patient groups so that trial data may be directly applied to clinical care without need for epidemiologic extrapolation.12 In view of the current abundance of data, a simple evidence-based guideline for statin therapy using the concepts of “what works?” and “in whom?” from completed randomized trials can be written without need for complex data modeling. As an example of this emerging approach, preventive cardiologists in the United States, Canada, and Europe have suggested the following list of five recommendations as a simple, easily understood guideline for the use of statin therapy in the prevention of cardiovascular disease that avoids controversy because it is based soundly on trial data13,14: 4. On the basis of high-quality randomized trial data, the use of nonstatin lipid-lowering agents for monotherapy or in combination with a statin should be limited during the wait for evidence that such an approach further reduces cardiovascular event rates in specific patient groups (AIM-HIGH [Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes], HPS2-THRIVE [Heart Protection Study 2-Treatment of HDL to Reduce the Incidence of Vascular Events], ACCORD [Action to Control Cardiovascular Risk in Diabetes], FIELD [Fenofibrate Intervention and Event Lowering in Diabetes]). In some cases this approach may be suboptimal, such as in patients who demonstrate statin intolerance or have familial hyperlipidemia and exceptionally high LDL cholesterol, or who have a risk of pancreatitis (see Chapter 45). Such patients can benefit from secondary evaluation by lipid specialists. This chapter reviews the epidemiologic and clinical trial evidence underlying risk markers and interventions to reduce atherothrombotic risk in three parts. The next section describes the conventional risk factors of smoking, hypertension, hyperlipidemia, and insulin resistance and diabetes, as well as general strategies for reducing risk related to these disorders. This section explores some of the issues and controversy surrounding the concept of the “metabolic syndrome.” It also reviews evidence describing the use of low-dose aspirin in primary prevention and briefly discusses the conceptual basis for the “polypill.” Not all coronary events occur in people with multiple traditional risk factors, however, and in some patients, abnormalities of inflammation, hemostasis, and/or thrombosis appear to contribute decisively. In particular, almost half of all MIs and strokes occur among persons without hyperlipidemia. Thus, subsequent to the section on conventional risk factors, another section reviews atherothrombotic risk markers, including hsCRP and other markers of inflammation (such as IL-1, IL-6, fibrinogen, and lipoprotein-associated phospholipase A2 [Lp-PLA2], as well as homocysteine and lipoprotein(a) [Lp(a)]). In each case, evidence is presented that describes whether these novel risk indicators add to risk prediction over and above that for conventional factors (see also Chapter 10). This section also reviews the use of hsCRP as part of the Reynolds risk score to evaluate global risk more effectively, further delineate the metabolic syndrome, and improve targeting of statin therapy. Also addressed is the use of direct plaque imaging as a method of risk detection; emerging concepts in the use of genetic biomarkers to help elucidate vascular risk and target novel therapies also are presented. The final section of the chapter addresses a series of environmental exposures and behavioral issues that have major impact on vascular health. This section reviews mental stress and depression and cardiovascular risk, as well as issues of diet, dietary supplements, obesity, exercise, and weight loss. This final section also reviews current evidence supporting moderate alcohol use, controversies surrounding postmenopausal estrogen, and issues of community-based and multiple risk factor intervention programs. With the exception of glucose intolerance and obesity, the prevalence of most cardiovascular risk factors has declined in the United States over the past 40 years. These favorable trends suggest that interventions to reduce risk can be highly effective when applied in appropriate settings, as evidenced not only by reductions in coronary disease but also by reductions in stroke. Prevention on an international scale is thus a feasible goal. Therefore targeting risk reduction by lifestyle modification and with proven medical therapies seems to be a sensible primary goal for outpatient preventive cardiovascular practice. Each of the following sections begins by focusing on the epidemiologic evidence linking the specific biomarker, exposure, or behavior to subsequent vascular risk. Then, in the spirit of “what works?” and “in whom?”, the randomized clinical trial evidence that supports modification of each risk marker is reviewed whenever possible. Chapter 44 takes a similar approach to the management of hypertension. In the setting of primary prevention, it is important to recognize that physicians do not measure biomarkers simply to predict risk. Rather, they do so to target therapy better and improve the lives of their patients. Thus, when considering the use of any biomarker for cardiovascular risk prediction in primary prevention, thoughtful clinicians should insist that two fundamental questions be answered affirmatively (see also Chapter 10): First, is there clear evidence that the biomarker of interest predicts future cardiovascular events independent of other risk markers? Second, is there clear evidence that persons identified by the biomarker of interest benefit from a therapy they otherwise would not have received? As described in this chapter, on the basis of current data, no imaging biomarker can answer these questions affirmatively, nor can measurement of a variety of plasma biomarkers such as Lp(a), homocysteine, or triglycerides. For cholesterol and for hsCRP, however, the answer to both of these questions is “yes,” because randomized, placebo-controlled trials have shown that patients identified by either of these biomarkers markedly benefit from statin therapy. These findings are of particular pathophysiologic interest in the modern view of atherothrombosis as resulting from interaction of hyperlipidemia and inflammation to initiate and accelerate all phases of the disease process (see also Chapter 41).15 Supporting this view, recent experimental work has suggested that the earliest deposition of cholesterol crystals triggers the interleukin-1-beta (IL-1β)–activating inflammasome, thus providing a key link between lipids, inflammation, and vascular disease.16 Other than advanced age, smoking remains the single most important risk factor for coronary artery disease. According to the 2010 Surgeon General’s Report17 cigarette consumption is the leading preventable cause of death and disease in the United States, accounting for approximately 443,000 deaths, or almost one of every five U.S. deaths, from smoking-related illnesses each year. An estimated 49,000 of these smoking-related deaths are the result of secondhand smoke exposure. Moreover, smoking has been estimated to cost the United States $96 billion in direct medical expenses and $97 billion in lost productivity annually.18 Smokers lose at least one decade of life expectancy, as compared with never-smokers.19 The risk of death from cigarette smoking continues to increase among women and the increased risks are now nearly identical for men and women.20 Compared with nonsmokers, smoking increases the risk of both coronary heart disease and stroke two- to fourfold. Ischemic heart disease underlies 35% to 40% of all smoking-related deaths, with an additional 8% attributable to secondhand smoke exposure. Cigarette smoking can promote vasoconstriction, resulting in a greater risk of developing symptomatic peripheral vascular disease and abdominal aortic aneurysm among smokers than nonsmokers. Secondhand smoke exposure also is associated with heart disease in nonsmoking adults. Nonsmokers exposed to secondhand smoke at home or work increase their heart disease risk by 25% to 30%. Breathing secondhand smoke has immediate harmful effects on the cardiovascular system that can increase the risk of heart attack, especially among those who already have heart disease. From a prevalence of adult smoking of 43% in 1964, the prevalence of adults smoking cigarettes fell to 19% in 2011,21 21.6% among males and 16.5% among females. Prevalence was lowest among non-Hispanic Asians (9.9%) and highest among non-Hispanic American Indians and Alaska natives (31.5%). By age, prevalence was lowest among adults 65 years of age and older (7.9%) and highest among those 25 to 44 years of age (22.1%). Prevalence was higher among adults living below the federal poverty level (29.0%) and among those reporting a disability (35.4%).22 The U.S. Healthy People 2020 initiative aims to reduce the national prevalence of cigarette smoking among adults to a target of 12%. The period 2005 to 2011 saw only a slight overall decline in current smoking prevalence, but the number of cigarettes smoked per day declined, as did the prevalence of current smoking occurring among adults 18 to 24 years of age. Among daily smokers overall, the proportion who smoked 30 cigarettes or more per day declined significantly from 12.6% in 2005 to 9.1% in 2011. This drop did not result from smoking cessation, however, because the proportion smoking 1 to 9 cigarettes per day increased significantly. For adults 18 to 24 years of age, current smoking prevalence declined from 24.4% to 18.9%. This age group, which had the highest prevalence in 2005, now has the lowest of any group younger than 65 years of age (Fig. 42-1). Consumption of tobacco products is increasing globally with the greatest increase in the developing world. Tobacco kills almost 6 million people each year, more than 5 million of whom are users and ex-users, and more than 600,000 of whom are nonsmokers exposed to secondhand smoke. This annual death toll could rise to more than 8 million by 2030.23,24 More than 80% of these deaths will be in low-income and middle-income countries. Tobacco caused 100 million deaths in the 20th century, and if current trends continue, it will cause up to 1 billion deaths in the 21st century. Landmark studies in the early 1950s first reported strong positive associations between cigarette smoke exposure and coronary heart disease. Over the next 50 years, an exceptionally consistent series of prospective studies documented the effects of smoking on coronary risk. The 1964 Surgeon General’s report reaffirmed the epidemiologic correlation, and by 1983 the Surgeon General had firmly established cigarette smoking as the leading avoidable cause of cardiovascular disease. Based largely on studies among men, the 1989 Surgeon General’s report showed that smoking doubles the incidence of coronary heart disease and increases coronary heart disease-related mortality by 50%, and that these risks increase with age and the number of cigarettes smoked. “Light” levels of smoking have a major impact on MI and all-cause mortality, even among smokers who do not report inhalation. In addition to MI, cigarette consumption directly relates to increased rates of sudden death, aortic aneurysm formation, symptomatic peripheral vascular disease, and ischemic stroke. Prospective evidence has linked cigarette consumption to an elevated risk of hemorrhagic stroke, including intracranial hemorrhage and subarachnoid hemorrhage, again in a dose-response manner. Continued smoking is also a major risk factor for recurrent MI. Even among nonsmokers, inhaled smoke, whether from passive exposure or from cigar or pipe consumption, increases coronary risk. Passive smoking exposure can cause endothelial vasodilator dysfunction in the coronary circulation as well as increased bronchial responsiveness and concomitant pulmonary dysfunction. There is no safe level of exposure to secondhand tobacco smoke. Women incur similar increases in the relative risk for coronary heart disease. Smoking acts synergistically with oral contraceptive agents, placing younger women taking these agents at even higher relative risk. Because of adverse synergy with oral contraceptives, young female smokers who take oral contraceptives have particularly elevated risks for premature coronary disease and stroke. Smoking is especially hazardous for women with diabetes. Beyond acute unfavorable effects on blood pressure and sympathetic tone and a reduction in myocardial oxygen supply, smoking contributes to the pathogenesis of atherothrombosis by several other mechanisms. Long-term smoking may enhance oxidation of LDL cholesterol and impair endothelium-dependent coronary artery vasodilation. This latter effect has been linked to dysfunctional endothelial nitric oxide biosynthesis with chronic as well as acute cigarette consumption. In addition, smoking has adverse hemostatic and inflammatory effects, including increases in levels of C-reactive protein (CRP), soluble intercellular adhesion molecule-1 (ICAM-1), fibrinogen, and homocysteine. Additionally, smoking is associated with spontaneous platelet aggregation, increased monocyte adhesion to endothelial cells, and adverse alterations in endothelium-derived fibrinolytic and antithrombotic factors, including tissue-type plasminogen activator and tissue pathway factor inhibitor. Compared with nonsmokers, smokers have an increased prevalence of coronary spasm and a reduced threshold for ventricular arrhythmias. Accruing evidence has suggested that insulin resistance represents an additional mechanistic link between smoking and premature atherosclerosis. Cessation of cigarette consumption overwhelmingly remains the single most important intervention in preventive cardiology. Although data from large-scale, randomized trials concerning the risk reduction associated with smoking cessation are limited, observational studies consistently demonstrate the clear benefits of smoking cessation. Smokers who quit reduce their excess risk of a coronary event by 50% within the first 2 years after cessation, with much of this benefit seen even within the first few months. Coronary heart disease risk falls substantially within 1 to 2 years of cessation, with the risk in former smokers approaching that in never-smokers after 3 to 5 years. Similarly, the risk of stroke decreases steadily after smoking cessation, with former smokers having the same stroke risk as in nonsmokers after 5 to 15 years. This benefit is much more rapidly realized than that seen with smoking and lung cancer. Moreover, the beneficial effect on coronary heart disease and mortality rates are seen even among elderly persons, supporting the idea that it is never too late to quit smoking for decreasing CHD coronary heart disease-associated risks (Fig. 42-2). These risk reductions equal or exceed those for other secondary prevention interventions that have received more attention from physicians and the pharmaceutical industry, including the use of aspirin, statins, beta-adrenergic blocking agents, and angiotensin-converting enzyme (ACE) inhibitors. Studies show that few people understand the specific health risks of tobacco use. Yet, among smokers who are aware of the dangers, most want to quit. Overall, approximately 69% of smokers in the United States want to quit completely,25 and in 2011, of current smokers and those who had quit during the preceding year, 51.8% had made a quit attempt for more than one day during the preceding year. Poor patient understanding of the importance of smoking cessation continues, particularly in the developing world. For example, a 2009 survey in China showed that only 37% of smokers knew that smoking caused coronary heart disease and only 17% knew it caused stroke. Substantial misunderstanding also surrounds, for example, the observation that smoking predicts better outcome after various reperfusion strategies (the so-called smoker’s paradox). Some researchers have regarded this effect as a “benefit” of smoking, but it probably reflects that smokers tend to undergo such procedures at a much younger age and hence have on average lower rates of comorbid illness. Clinical practice guidelines recognize tobacco dependence as a chronic condition that often requires repeated interventions. Nevertheless, effective evidence-based treatments do exist. Multiple attempts may be necessary, but smokers can and do quit smoking. In fact, since 2002, the number of former smokers has exceeded the number of current smokers.25 Decades of research have documented the effectiveness of a broad strategy involving multifaceted interventions. A number of individual-level treatments have proved effective for smokers who want help to quit. These approaches include brief clinical interventions (e.g., a physician taking 10 minutes or less to deliver advice and assistance about quitting); counseling (e.g., individual, group, or telephone counseling); behavioral cessation therapies (e.g., training in problem solving); and treatments with more person-to-person contact and intensity. Cessation medications found to be effective for treating tobacco dependence include nicotine replacement products, either over-the-counter (e.g., nicotine patch, gum, lozenge) or prescription (e.g., nicotine inhaler, nasal spray), and prescription non-nicotine medications such as bupropion SR, varenicline tartrate, or new agents such as cytisine.26 A 2008 Cochrane review found no overall difference in the effectiveness of the various forms of nicotine replacement therapy. The combination of medication and counseling is more effective for smoking cessation than either medication or counseling alone, with multiple counseling sessions increasing the success rate. Reductions in smoking from any mechanism improve health outcomes, particularly when linked to lifestyle changes, including exercise and dietary control. Trials of nicotine replacement therapy using transdermal nicotine or nicotine chewing gum increase abstention rates after cessation. Such pharmacologic programs, as well as physician-guided counseling, are cost-effective and should be provided as standard prevention services. Smoking low tar or low nicotine cigarettes rather than regular cigarettes appears to have little effect on reducing the risk of coronary heart disease. Although the elevated cardiovascular risks associated with smoking decrease significantly after cessation, the risk for development of cancer of the lungs, pancreas, or stomach persists for more than a decade, as does that for chronic obstructive pulmonary disease. Smoking cessation has clear benefit, but smoking reduction alone appears to have only a marginal effect. A number of evidence-based methods for population-based smoking cessation also exist. The World Health Organization (WHO) Framework Convention on Tobacco Control, which began in 2005, has been one of the most widely accepted treaties in the history of the United Nations, with more than 170 parties covering 87% of the world’s population. In 2008, the WHO introduced a package of evidence-based tobacco control measures to help countries implement the WHO Framework Convention. Entitled MPOWER, the measures include increasing prices of tobacco products; anti-tobacco media campaigns featuring graphic personal stories on the adverse health impacts of smoking; implementing smoke-free laws for workplaces and public places; barrier-free access to help quitting; and enforcing restrictions on tobacco advertising, promotion, and sponsorship.24 Studies carried out after the implementation of pictorial package warnings in a number of countries consistently have shown that they significantly increase people’s awareness of the harms of tobacco use. Increasing tobacco taxes have also been a highly effective way to reduce tobacco use. Accomplishing the goal of Healthy People 2020 to reduce the U.S. national prevalence of cigarette smoking among adults to a target of 12% will require more extensive implementation of evidence-based tobacco control interventions. Only two states have funded tobacco control programs at Centers for Disease Control and Prevention (CDC)-recommended levels, whereas 27 states are funded at less than a quarter of these levels. State funding in tobacco control programs has in fact decreased during the last five years. Good monitoring also is important to track the extent and character of the tobacco epidemic and indicates how best to tailor policies. Several advances in tobacco control have occurred recently in the United States.27 Four new laws have reinvigorated the national effort, including implementation of the 2009 Family Smoking Prevention and Tobacco Control Act, which granted the U.S. Food and Drug Administration (FDA) the authority to regulate the manufacture, distribution, and marketing of tobacco products; the Children’s Health Insurance Reauthorization Act; the Prevent All Cigarette Trafficking Act; and the Patient Protection and Affordable Care Act. These laws have granted federal agencies more authority and funding to regulate tobacco products, decrease youth access to tobacco, and increase access to treatment programs. In 2010, the U.S. Department of Health and Human Services presented its first national strategic plan for tobacco control, with 21 action steps involving coverage of cessation treatment, reduction of youth access to tobacco, investments in state and local tobacco control initiatives, and communication efforts to engage the public. A federal mass media campaign began in early 2012, using graphic personal stories on the adverse health impact of smoking. In the context of these renewed efforts, however, the low success rates in smoking cessation continue to challenge clinicians. Preventing smoking in the first place should receive greater emphasis. Community education and physician-based primary prevention remain the most important components of any smoking reduction strategy. Elevated blood pressure is a major risk factor for coronary heart disease, heart failure, cerebrovascular disease, peripheral arterial disease, renal failure, atrial fibrillation, and total mortality, as well as loss of cognitive function and increased incidence of dementia (see also Chapters 1, 43, and 44). The degree of blood pressure lowering relates linearly to risk reduction. Observational data indicate that death from both coronary heart disease and stroke increases progressively from blood pressure levels as low as 115 mm Hg systolic and 75 mm Hg diastolic. For patients 40 to 70 years of age, each increment of 20 mm Hg in systolic blood pressure or 10 mm Hg in diastolic blood pressure doubles the risk of cardiovascular disease across a blood pressure range of 115/75 to 185/115 mm Hg. Prehypertension, defined as systolic blood pressure between 120 to 139 mm Hg or diastolic blood pressure between 80 to 89 mm Hg, is associated with nearly twice the risk of MI and stroke in women compared with normal blood pressure. Hypertension often confers silent cardiovascular risk, and its prevalence is steadily increasing in the United States and worldwide. Approximately 78 million—or 1 in 3—adults in the United States have high blood pressure, defined as systolic blood pressure of 140 mm Hg or greater or diastolic blood pressure of 90 mm Hg or greater or taking antihypertensive medicine.28,29 Men have a higher percentage of hypertension than women until the age of 45 years; between 45 and 64 years of age, men and women have similar percentages of hypertension; and after 64 years of age, a higher percentage of women have diagnosed hypertension than men (Fig. 42-3). The prevalence of hypertension increases markedly with age in all races and ethnicities. The age-adjusted prevalence of hypertension (both diagnosed and undiagnosed) in the period 2003 to 2006 was 75% for older women and 65% for older men. The prevalence of hypertension in the United States varies geographically, with the percentage of adults who had been told they were hypertensive ranging from 22.9% in Utah to 40.1% in Alabama. Disparities in hypertension by racial and ethnic groups persist (Fig. 42-4). Blacks develop high blood pressure more often, and at an earlier age, than do whites and Mexican Americans and have higher average blood pressure levels. Among blacks, more women than men have hypertension. Data from the National Health and Nutrition Survey (NHANES) indicate that from 1988 to 1994 through 1999 to 2002, the prevalence of high blood pressure in adults increased from 35.9% to 41.4% among blacks, and it was particularly high among black women at 44.0%. By their 60s, more than 80% of non-Hispanic black women are classified as being hypertensive.30 The prevalence of hypertension in blacks in the United States is among the highest in the world, and is increasing. According to data from NHANES 2001 to 2006, among those who were hypertensive, non-Hispanic blacks and Mexican Americans had 40% higher odds of having uncontrolled blood pressure than in non-Hispanic whites.31 As a result, compared with whites, blacks have a 1.3 times greater rate of nonfatal stroke, a 1.8 times greater rate of fatal stroke, a 1.5 times greater rate of death attributable to heart disease, and a 4.2 times greater rate of end-stage kidney disease. Within the black community, rates of hypertension vary substantially, with persons with the highest rates more likely to be middle-aged or older, less educated, overweight or obese, and physically inactive and more likely to have diabetes mellitus, but those with uncontrolled high blood pressure who do not take antihypertensive medication tending to be male and younger and to have infrequent contact with a physician. Data from NHANES 2007 to 2010 indicate that 6% of U.S. adults have undiagnosed high blood pressure. Of those with hypertension who are 20 years of age or older, 81.5% were aware they were hypertensive; 74.9% were under current treatment, 52.5% had their hypertension under control, and 47.5% did not achieve control.29 Rates of control differ substantially by ethnic and racial groups (Table 42-1). The rates of control were lower in Mexican Americans (39.3%) than in non-Hispanic whites (54.9%) and non-Hispanic blacks (47.6%). Findings from the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study support the success of efforts to raise awareness of prevalent hypertension and importance of receiving treatment among blacks but also show substantial persistent racial disparities with regard to the control of blood pressure, with the odds of control being 27% lower in blacks than in whites. No geographic disparities were found in hypertension awareness, treatment, and disease control.32 These data indicate that most people know their hypertension status, but 47.5% do not have their hypertension controlled.28 Awareness and treatment rates of hypertension have increased substantially. The control rates increased in both sexes, in non-Hispanic blacks, in Mexican Americans, and in those 60 years of age and older. Yet, although control of hypertension improved, control rates remain low, particularly among older patients (Fig. 42-5). Data from the Framingham Heart Study show that among those 80 years of age or older, only 38% of men and 23% of women had blood pressures that met targets set forth in the National High Blood Pressure Education Program’s clinical guidelines. Similarly, data from the Women’s Health Initiative (WHI) observational study of nearly 100,000 postmenopausal women across the country indicate that despite similar treatment rates, older women maintain especially poor hypertensive control. Among U.S. adults with hypertension, 8.9% meet the criteria for resistant hypertension (blood pressure ≥140/90 mm Hg, despite reported use of antihypertensive medications from three different drug classes or drugs from four or more antihypertensive drug classes regardless of blood pressure). This segment represents 12.8% of the population taking antihypertensive medications.33 On the other end of the spectrum, data from NHANES 1999 to 2006 indicate that 29.7% of U.S. adults 20 years or older have prehypertension, defined as untreated systolic blood pressure of 120 to 139 mm Hg or untreated diastolic blood pressure of 80 to 89 mm Hg and not having been told on two occasions by a physician or other health professional that they have hypertension. Prehypertension is associated with elevated relative and absolute risks for cardiovascular outcomes across the age spectrum, including an association with incident stroke, particularly in nonelderly persons and for those with blood pressure values in the higher prehypertension range.34 In the United States, the prevalence of hypertension is increasing across all race/ethnicity and age groups. By 2030, the prevalence of hypertension is projected to increase 7.2% from 2013 estimates. Costs directly attributable to high blood pressure for the United States total almost $131 billion annually in direct medical expenses and $25 billion in lost productivity,35 and projections show that by 2030, the total cost of high blood pressure will increase to an estimated $343 billion.29 Worldwide almost 1 billion adults have hypertension, 333 million in economically developed countries and 639 million in developing countries. By 2025, the total number of adults with hypertension is anticipated to top 1.5 billion. Hypertension causes 7.6 million premature deaths worldwide annually, with 80% of this burden occurring in low-income and middle-income countries.36 Approximately three quarters of persons with hypertension (639 million) live in developing countries with limited health resources, and where people have a very low awareness of hypertension and poor blood pressure control.37,38 The proportion of people with hypertension who have their hypertension under control in some countries, such as rural Ecuador, is as low as 0.3%. This high prevalence of hypertension and poor hypertension control contribute importantly to the rising epidemic of cardiovascular disease in developing countries. Several hypertension risk factors seem to be more common in developing countries than in developed regions, including urbanization, aging of the population, changes to dietary habits, and social stress. High illiteracy rates, limited access to health facilities, poor dietary habits, poverty, and high costs of drugs all contribute to poor blood pressure control.37 Numerous risk factors and markers for development of hypertension have been identified, including increasing age, ethnicity, family history of hypertension, genetic factors, lower education and socioeconomic status, greater weight, lower physical activity, tobacco use, psychosocial stressors, sleep apnea, and dietary factors (including increased dietary fats, higher sodium intake, lower potassium intake, and excessive alcohol intake). Data suggest that controlling dietary and lifestyle risk factors can prevent a large proportion of incident hypertension in women.39–41 Young women who adopt healthy practices such as maintaining normal weight, eating a healthful diet, exercising daily, drinking a moderate amount of alcohol, and limiting use of over-the-counter analgesics can greatly reduce the risk of hypertension. Patients with concomitant chronic kidney disease (estimated glomerular filtration rate below 60 mL/m2) constitute a high-risk group for focused blood pressure treatment, both for the prevention of cardiovascular disease and to slow progression to end-stage renal disease. Patients with obesity, the metabolic syndrome, and diabetes also represent high-risk groups for treatment. High blood pressure occurs in more than two thirds of patients with type 2 diabetes, and its development coincides with the development of hyperglycemia.42
Risk Markers and the Primary Prevention of Cardiovascular Disease
Rethinking Core Approaches to Primary Prevention
What Works and in Whom? a Simple Evidence-Based Alternative to the Prevention of Cardiovascular Disease
Merging Epidemiology and Randomized Trial Evidence: Why Measure Risk Factors?
Conventional Risk Markers and Their Interventions
Smoking
Interventions for Smoking Cessation
Hypertension
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Risk Markers and the Primary Prevention of Cardiovascular Disease
42