Risk factors for developing systemic-to-pulmonary artery collaterals (SPCs) in hypoplastic left heart syndrome (HLHS) are unknown. We performed a retrospective case-control study to identify risk factors for developing a profuse SPC burden in HLHS. Angiograms of 439 patients with HLHS (performed <2 years of age) were reviewed using a previously published angiographic grading scale to identify cases (profuse SPC burden, n = 20) and controls (no or minimal SPC burden, n = 35). In univariate analyses, profuse SPC burden was associated with mitral atresia and aortic atresia subtype (MA/AA) (65% vs 14%, p <0.0001), use of a Sano shunt (70% vs 37%, p = 0.03), longer log-transformed durations of intensive care unit stay (p = 0.02), hospital stay (p = 0.002), pleural drainage (p = 0.008) after stage 1 palliation, lower oxygen saturation at discharge after stage 1 palliation (82 ± 4 vs 85 ± 4%, p = 0.03), and a history of severe shunt obstruction (37% vs 11%, p = 0.04). In a multivariate logistic regression model, profuse SPC burden was associated with MA/AA subtype (odds ratio 6.6), Sano shunt type (odds ratio 8.6), and log-transformed duration of hospital stay after stage 1 (odds ratio 7.9, model p <0.0001, area under the curve 0.88). Nonassociated parameters included fetal aortic valve dilation, severe cyanotic episodes, number of days with open sternum or number of additional exploratory thoracotomies after stage 1 palliation, pulmonary vein stenosis, and restrictive atrial septal defect. In conclusion, in the present case-control study of patients with HLHS, the development of a profuse SPC burden was associated with MA/AA subtype, Sano shunt type, and longer duration of hospital stay after stage 1 palliation.
Systemic-to-pulmonary artery collaterals (SPCs) are common in patients with single ventricle physiology and are seen in nearly 2/3 of patients after a bidirectional Glenn operation and in approximately 1/2 of patients after a Fontan operation. A high SPC burden has been linked with increased morbidity ; however, the risk factors and pathogenic mechanisms responsible for the development of SPCs in this patient population remain poorly characterized. Chronic hypoxemia, diminished pulmonary blood flow, surgery-related scar formation, branch pulmonary artery stenosis, inflammation, and abnormal pulmonary artery flow patterns have all been hypothesized to contribute to their formation. We have previously shown that children with hypoplastic left heart syndrome (HLHS) have a particularly high risk of developing SPCs. Accordingly, the purpose of this study was to identify risk factors for the development of profuse SPCs in children with HLHS.
Methods
A retrospective case-control study design was used. The Scientific Review Committee of the department of cardiology and the Boston Children’s Hospital Committee on Clinical Investigation gave permission for a retrospective review of existing data.
Patients with HLHS treated from January 2000 through May 2012 at Boston Children’s Hospital were identified, and those who had x-ray angiography for assessment of SPCs during the first 2 years of life, including ≥1 evaluation beyond 1 year of life, were eligible for inclusion. Catheterization reports and angiograms for these patients were screened to identify patients who preliminarily qualified as either cases (profuse SPC burden) or controls (no or minimal SPC burden). The angiograms of these patients were then reviewed in detail by an expert in interventional cardiology (blinded to other clinical data) who graded the SPC burden using a previously described angiographic grading scale (none or mild, moderate, or profuse). Cases were defined as patients who had ≥1 angiogram demonstrating profuse SPC burden, and controls as those who consistently demonstrated no or minimal SPC burden before age 2 years. Patients who did not survive to age 1 year and those with moderate SPC burden were excluded from both groups. To assess interrater reliability, a second expert in interventional cardiology independently examined the angiograms on 30 randomly selected study patients and classified them into cases and controls.
For cases and controls, clinical, surgical, cardiac catheterization, and echocardiographic data were abstracted from the medical record. Data collected included details of anatomic diagnosis, surgical procedure, postoperative course, hemodynamic and angiographic data, episodes of severe cyanosis (saturation <70% for a period >24 hours), and growth parameters (weight-for-age z score at 18 months). Echocardiograms were reviewed for qualitatively greater than mild ventricular dysfunction, greater than mild atrioventricular valve regurgitation, and restricted flow across the atrial septum (mean gradient >3 mm Hg).
Statistical analyses were performed using commercially available software (STATA version 12.0, StataCorp, College Station, Texas). Cases and controls were compared for differences in the prevalence of factors that have been hypothesized to promote the development of SPCs ( Table 1 ). Transformations to continuous variables were applied as appropriate to remedy skewness. Unadjusted, univariate associations were assessed using a Student’s t test, Wilcoxon rank-sum test, or Fisher’s exact test, as appropriate. Multivariate logistic regression analysis with forward selection was used to analyze the statistical significance of associations between predictor variables and case or control status. Variables demonstrating univariate associations with p <0.05 qualified for inclusion in the multivariate model. Analyses of receiver operating characteristics were used to evaluate the fit of the multivariate models. Cases and controls were also compared for differences in outcomes including postoperative courses after the bidirectional Glenn and Fontan operations and recent clinical status including prevalence of greater than mild ventricular dysfunction or atrioventricular valve regurgitation and growth failure.
| Variable | Cases (n = 20) | Controls (n = 35) | p |
|---|---|---|---|
| Type of HLHS | 0.001 | ||
| Mitral stenosis, aortic stenosis | 4 (20) | 18 (51) | |
| Mitral atresia, aortic atresia | 13 (65) | 5 (15) | |
| Mitral stenosis, aortic atresia | 3 (15) | 6 (17) | |
| Others | 0 (0) | 6 (17) | |
| Fetal aortic valve balloon angioplasty | 2 (10) | 3 (14) | 0.9 |
| Type of stage 1 surgical palliation | 0.03 | ||
| Blalock-Taussig shunt | 6 (30) | 22 (63) | |
| Right ventricle-to-pulmonary artery type shunt | 14 (70) | 13 (37) | |
| Postoperative course after stage 1 palliation | |||
| Days with open sternum | 4 (2–15) | 3 (0–9) | 0.02 |
| Number of surgical explorations | 1 (0–1) | 0 (0–1) | 0.02 |
| Wound infection | 2 (10) | 2 (6) | 0.6 |
| Days of pleural tube drainage | 5 (3–10) | 3 (1–11) | 0.008 |
| Days in intensive care unit | 13 (3–78) | 9 (3–24) | 0.02 |
| Days in hospital | 30 (12–84) | 19 (8–139) | 0.002 |
| Pulse oximetry at discharge | 82 (72–88) | 84 (77–94) | 0.02 |
| Severe shunt obstruction | 7 (37) | 4 (11) | 0.04 |
| Severe cyanotic episode (saturation <70% for >24 h) | 5 (25) | 5 (14) | 0.5 |
| Pulmonary vein stenosis | 2 (10) | 3 (9) | 0.9 |
| Restrictive atrial septum after stage 1 palliation | 3 (15) | 11 (31) | 0.3 |
Results
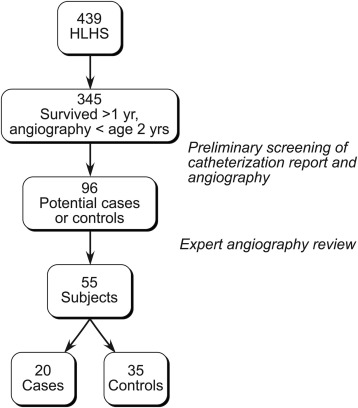
The process for case and control subject selection is summarized in Figure 1 . From January 2000 to July 2012, 439 patients with HLHS underwent treatment at Boston Children’s Hospital. Of these, 395 survived beyond 1 year of age and had angiography performed under 2 years of age, with ≥1 evaluation beyond 1 year of age. An initial screen identified 96 patients who preliminarily qualified as either cases (profuse SPC burden) or controls (no or minimal SPC burden). Angiograms of these 96 patients were then reviewed in detail by an expert in interventional cardiology who classified 20 as cases and 35 as controls. On a random sample of 30 patients, interrater reliability in assigning cases and controls was moderately good (agreement 80%, κ statistic 0.60, p <0.0002). The proportions of patients born before 2005 were similar between cases (55%) and controls (37%, p = 0.3). Patients classified as cases were first noted to have profuse SPC burden at a median age of 5 months (range 3 to 21) and were more likely to have undergone coil occlusion of SPCs under 2 years of age (100% vs 11%, p <0.0001).
Results of univariate comparisons between cases and controls are listed in Table 1 . The development of a profuse SPC burden was significantly associated with mitral atresia with aortic atresia (MA/AA) anatomic subtype; right ventricle-to-pulmonary artery type (Sano) shunt; longer log-transformed durations of intensive care unit stay, hospital stay, and pleural drainage after stage 1 palliation; lower oxygen saturation at discharge after stage 1 palliation; and history of severe shunt obstruction. Nonassociated parameters included a history of fetal aortic valve dilation, a history of severe cyanotic episodes (saturation <70% for >24 hours), the number of days with open sternum, the number of surgical explorations after stage 1 palliation, a history of pulmonary vein stenosis (identified by echocardiography or angiography before age 2 years), and the presence of a restrictive atrial septal defect (mean gradient by echocardiography >3 mm Hg) after stage 1 palliation.
Results of multivariate logistic regression analysis are listed in Table 2 . In a model controlling for surgical era, an MA/AA anatomic subtype, right ventricle-to-pulmonary artery type shunt, and log-transformed duration of hospital stay after stage 1 remained significantly associated with profuse SPC burden. Analysis of receiver operating characteristics confirmed a good model fit (area under the curve 0.88, Figure 2 ). Of these 3 factors that were independently associated with profuse SPC burden, the presence of a greater number of factors was associated with a greater likelihood of profuse SPC burden, as shown in Figure 3 . For this analysis, hospital stay after stage 1 palliation was analyzed as a binary variable using a cut-off value of 48 days (determined using receiver operating characteristics analysis of log-transformed hospital stay and case or control designation).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


