, Marilyn J. Siegel2, Tomasz Miszalski-Jamka3, 4 and Robert Pelberg1
(1)
The Christ Hospital Heart and Vascular Center of Greater Cincinnati, The Lindner Center for Research and Education, Cincinnati, OH, USA
(2)
Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis, Missouri, USA
(3)
Department of Clinical Radiology and Imaging Diagnostics, 4th Military Hospital, Wrocław, Poland
(4)
Center for Diagnosis Prevention and Telemedicine, John Paul II Hospital, Kraków, Poland
Abstract
Congenital pulmonary valve abnormalities encompass a spectrum of anomalies that result in right ventricular outflow tract (RVOT) obstruction. Based on the level of obstruction, there are three possible variants: (a) valvular, (b) subvalvular (infundibular stenosis), and (c) supravalvular.
14.1 Pulmonary Valve Abnormalities
Congenital pulmonary valve abnormalities encompass a spectrum of anomalies that result in right ventricular outflow tract (RVOT) obstruction. Based on the level of obstruction, there are three possible variants: (a) valvular, (b) subvalvular (infundibular stenosis), and (c) supravalvular.
14.1.1 Valvular Pulmonary Stenosis
Valvular pulmonary stenosis refers to a congenital narrowing at the level of the pulmonary valve. It is the most common cause of RVOT obstruction, accounting for 7–12 % of all congenital heart diseases [1].
Morphologic features of the stenotic pulmonary valve vary and include (a) the dome-shaped valve and (b) the dysplastic myxomatous valve. The most common morphology is the dome-shaped, tricuspid pulmonary valve with thickened, fused commissures, a narrow central opening (pinhole to several millimeters), preserved leaflet mobility, and poststenotic dilatation of the pulmonary artery. The valve annulus is usually abnormal and lacks fibrous support. The dome-like appearance results from protrusion of the fused valve leaflets into the pulmonary artery [2]. The main pulmonary artery is dilated in almost all cases, related to a high-velocity jet across the stenotic valve (Fig. 14.1). Relatively uncommon variants of valvular stenosis include unicommissural, bicuspid, and quadricuspid valves (Figs. 14.2 and 14.3).
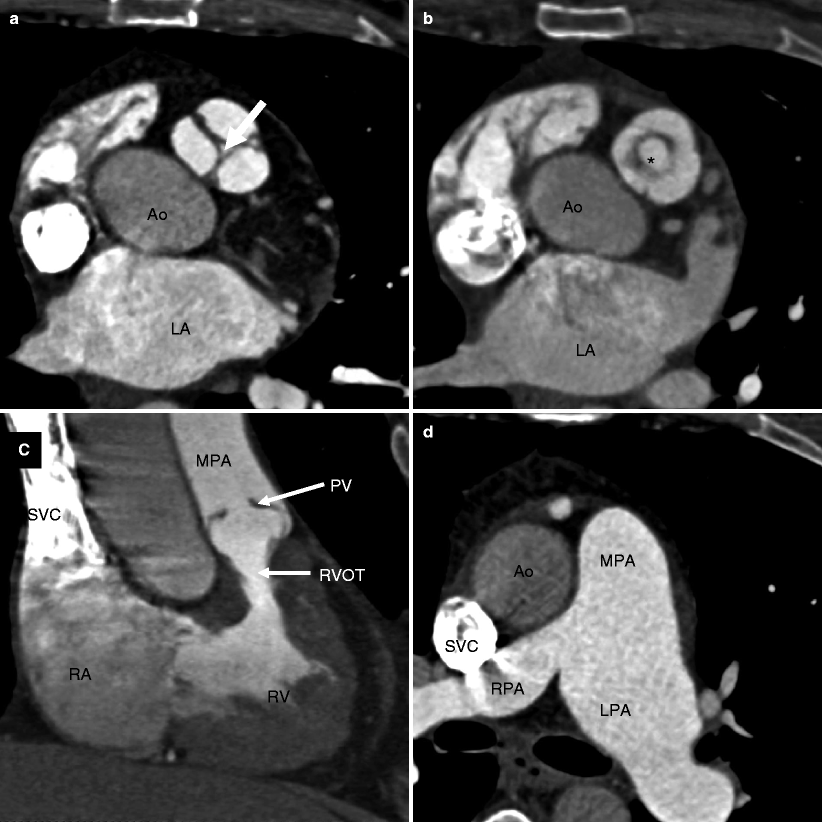
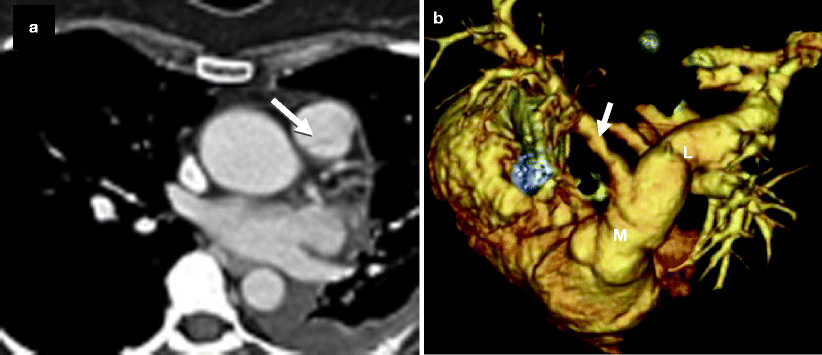
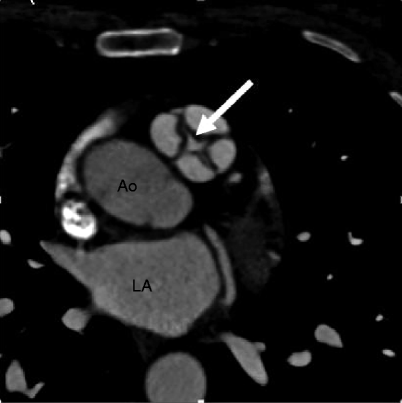

Fig. 14.1
Valvular pulmonary stenosis. Panel (a) is an axial computed tomogram (CT) showing a trileaflet pulmonary valve with thickened commissures and a small central orifice (arrow). Panel (b) is a CT at a different anatomic level showing the stenotic pulmonary valve orifice (asterisk). Panel (c) depicts a coronal reformatted view showing the thickened and domed pulmonic valve (PV) and compensatory right ventricular (RV) hypertrophy with narrowing of the right ventricular outflow tract (RVOT). Panel (d) is an axial CT at a higher anatomic plane showing the dilated main (MPA) and left pulmonary (LPA) arteries. RPA right pulmonary artery, SVC superior vena cava, Ao aorta, RA right atrium

Fig. 14.2
Bicuspid pulmonary valve. Panel (a) is an axial scan showing a pulmonic valve with two asymmetric cusps. The arrow points to the raphe between the two cusps. Panel (b) is a volume-rendered reconstruction showing the dilated main (M) and left (L) pulmonary arteries. For comparison, the right pulmonary artery (arrow) is of normal size

Fig. 14.3
Quadricuspid pulmonic valve. An axial scan showing four pulmonary valve leaflets of equal size with commissural thickening and incomplete coaptation centrally (arrow). Ao aorta, LA left atrium
Pulmonary valve dysplasia is characterized by thickened, redundant valvular leaflets, minimal or no commissural fusion, poor or absent leaflet mobility, and lack of poststenotic dilatation of the pulmonary artery. The obstruction is related to myxomatous replacement of the valve cusps and hypoplasia of the pulmonary valve ring.
The stenotic (dome-shaped or dysplastic) pulmonary valve may be an isolated lesion, although it is often associated with peripheral pulmonary stenosis, ventricular and atrial septal defects, tetralogy of Fallot, and Noonan syndrome (phenotypically Turner syndrome and genotypically XX or XY) [3]. Noonan patients with PTPN11 mutations are more likely to have pulmonary valve stenosis than those without this mutation [3].
The hemodynamic consequence of pulmonic stenosis is proportional to the severity of the obstruction. Patients with moderate pulmonic stenosis are often detected in infancy and childhood, but they may be asymptomatic until adulthood. Eventually, the valvular obstruction induces changes in the right ventricle muscle, which hypertrophies, especially in the infundibular region, leading to ventricular dilatation, systolic dysfunction, and right-sided heart failure. Symptoms then include fatigue, dyspnea on exertion, cyanosis, and right heart failure. Progression of moderate pulmonary stenosis is not uncommon; mild pulmonary stenosis is rarely progressive [2].
Surgical intervention is necessary in symptomatic patients. Percutaneous balloon pulmonary valvotomy is widely used in the dome-shaped stenotic pulmonary valve. Recently, percutaneous valve replacement has been used in patients with dome-shaped stenotic valves [1]. Surgery is preferred in patients with dysplastic pulmonary valves and also in those with complications of pulmonary regurgitation, subvalvular stenosis, and severe tricuspid regurgitation [3]. The surgical approach includes a partial or total valvotomy, a transannular patch, and valve replacement. The latter is performed when there is significant valvular regurgitation. In addition, pulmonary homograft replacement may be needed. After interventions, patients should be followed for residual RVOT obstruction, pulmonary valve regurgitation, right ventricular dilatation and systolic dysfunction, and tricuspid valve regurgitation [2, 3].
14.1.2 Infundibular or Subvalvular Stenosis
Infundibular stenosis refers to obstruction at the level of the right ventricular infundibulum within the body of the ventricle, as opposed to obstruction at the pulmonary valve, pulmonary artery, or its branches (Fig. 14.4). Primary infundibular stenosis is a rare cardiac malformation accounting for a small minority of RVOT obstruction.
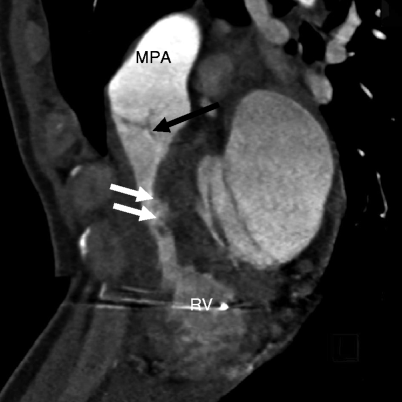

Fig. 14.4
Subinfundibular pulmonary stenosis. Here is a reformatted image showing a narrowed infundibulum (white arrows). The pulmonic valve (black arrow) and main pulmonary artery (MPA) are normal. RV right ventricle
There are two forms of infundibular stenosis. The first type is associated with a thickened muscular/fibrous infundibulum that narrows the right ventricular outlet. The narrowed area may be short or long and may be located immediately below the pulmonary valve or lower in the outflow tract. The second type is associated with stenosis of the infundibulum due to a fibrous or muscle band at the junction of the main cavity of the right ventricle and the infundibulum. The band divides the cavity into a high-pressure proximal and low-pressure distal chamber. This is referred to as a “double-chambered right ventricle” [3].
The anomalous muscle bundle may be an anomalous septoparietal band, anomalous apical shelf, or abnormal moderator band [1]. It typically extends between the interventricular septum (in the area of septomarginal trabeculation) and the anterior wall of the right ventricle and may take a diagonal or horizontal course through the ventricle [4]. Double-chambered right ventricles are rare, accounting for 1 % of congenital heart diseases [4]. Infundibular muscular obstruction is associated with ventricular septal defect, atrial septal defect, valvular pulmonary stenosis, tetralogy of Fallot, and double-outlet right ventricle [3].
The hemodynamic consequence of the obstruction is elevated pressure within the right ventricular cavity. This high pressure is limited to the portion proximal or the infundibulum. Lower or even normal pressures are present beyond the obstruction site. RVOT obstruction usually progresses over time. Long-term complications include compensatory right ventricle hypertrophy with elevated end-diastolic pressures, elevated right atrial pressure and dilatation, and eventually right-sided heart failure. Complete or partial spontaneous closure of an associated ventricular septal defect may worsen the RVOT obstruction [3]. Most patients are diagnosed during childhood or adolescence and undergo surgical repair. However, unrepaired subinfundibular stenosis has been reported in adults [5–7].
Nonmuscular causes of infundibular obstruction are rare and include a prominent or displaced tricuspid valve, fibrous tags arising in the inferior vena cava or coronary sinus, sinus of Valsalva aneurysm, and aneurysm of the membranous portion of the interventricular septum [8–12].
Surgical intervention is considered in patients who are symptomatic or who have high-pressure gradients across the obstruction. Treatment of muscle bands involves resection of the muscle bundles and repair of associated lesions. Most patients do well after surgery and recurrence of obstruction is rare [13, 14]. Interventions for other subinfundibular obstructions include percutaneous balloon dilatation, stenting, and percutaneous alcohol ablation [15–17].
14.1.3 Supravalvular Stenosis
Supravalvular stenosis refers to obstruction at the level of the main, major, or peripheral pulmonary arteries [3]. Supravalvular stenosis is a rare abnormality that occurs in 2–3 % of patients with congenital heart disease (Fig. 14.5).
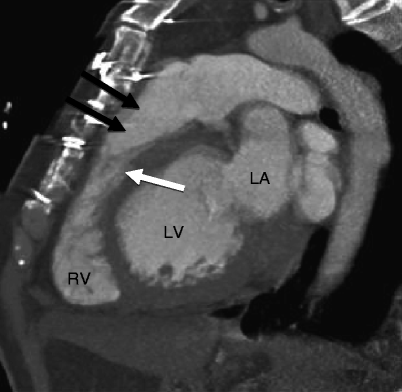

Fig. 14.5
Supravalvular pulmonary stenosis in a patient with Williams syndrome. The patient had prior surgery for repair of a stenotic bicuspid aortic valve. This sagittal scan shows a narrowing of the main pulmonary artery (black arrows) above the level of the pulmonic valve (white arrow). Left ventricular hypertrophy is related to recurrence of aortic valve stenosis
Supravalvular stenosis can involve the trunk, main, or peripheral pulmonary arteries. It can be isolated or involve multiple areas in one or both lungs [3, 18–20]. Based on the location, stenoses may be divided into four types. Type I is characterized by a single central stenosis (main, right, or left pulmonary artery). In type II, the stenosis is located at the bifurcation of the pulmonary arterial trunk, extending to the origins of the right and left pulmonary arteries. The type III stenoses are peripheral only. In type IV, there is combined central and peripheral stenoses [18]. Type I pulmonary supravalvular stenosis with central stenosis is most often associated with dilatation of the poststenotic portion of pulmonary. Poststenotic dilatation, if it occurs, is minimal in type II obstruction and is absent in type III stenosis. Most supravalvular stenoses are due to intimal or muscular proliferation but may be also produced by a pulmonary artery membrane or aneurysm [1].
A hemodynamically significant lesion is defined as ≥50 % diameter narrowing. In general, adults are asymptomatic unless they have high right ventricular pressures or associated cardiac disease. They may come to clinical attention because of a murmur, or they may be referred for computed tomographic angiography of a dilated main pulmonary artery found on a plain radiography [3]. Symptoms can mimic those of pulmonary thromboembolic disease and include fatigue and dyspnea.
Supravalvular stenosis is frequently associated with Noonan syndrome, Williams syndrome, tetralogy of Fallot, trisomy 21 syndrome, and Ehlers–Danlos syndrome and may be detected incidentally during evaluation of these conditions. These lesions, particularly peripheral stenoses, may be progressive.
Treatment of hemodynamically significant focal main, branch, or peripheral pulmonary artery stenoses is usually performed by balloon angioplasty. Patients with main or bifurcation stenoses who are not candidates for percutaneous intervention or who fail percutaneous angioplasty may require surgical dilatation or resection of the stenotic area to relieve right ventricular hypertension. Peripheral stenotic segments are usually not amenable to surgical intervention [3]. Recurrence of stenosis is not uncommon, particularly in peripherally stenotic lesions [3].
14.1.4 Cardiac Computed Tomography (CT) in the Evaluation of Pulmonary Valve Abnormalities
CT can be a useful noninvasive modality when echocardiography and magnetic resonance do not provide sufficient data. It is especially useful for assessment of peripheral pulmonary artery stenosis [3, 21, 22]. CT findings of pulmonary valve obstruction include a narrowed or stenotic segment with or without poststenotic dilatation of the distal arterial segments, a jet of contrast material extending across a narrowed segment, and right ventricular hypertrophy. The elevated right ventricular pressure and volume may cause bowing of the interventricular septum to the left.
In valvular pulmonic stenosis, the main and left pulmonary arteries are dilated (see Figs. 14.1 panel d and 14.2 panel b). The turbulent jet across the valve extends preferentially into the main and left pulmonary arteries, sparing the right pulmonary artery. Poststenotic dilatation is less common with dysplastic valves than it is with the dome-shaped mobile pulmonic valves. Cine images may show decreased mobility of the valve leaflets.
CT assessments should include evaluation of (a) the morphology, wall thickness, size, and systolic function of right ventricle; (b) the morphology and size of the right atrium; (c) the morphology, size, and function of the pulmonary valve, including the systolic orifice area, and the presence of associated pulmonary valvular regurgitation; (d) the presence, site, morphology, and size of infundibular and subinfundibular obstruction; (e) the presence, size, morphology, and site of pulmonary artery obstruction; (f) the anatomy, size, and function of the tricuspid valve; and (g) coexisting anomalies. See Table 14.1. CT may also depict bronchial compression by the dilated pulmonary arteries, which cannot be demonstrated by echocardiography. In addition, rare complications such as compression of contiguous cardiac structures including compression of left main coronary artery may be detected with CT [3].
Table 14.1
Evaluation of right ventricular outflow tract obstruction with computed tomography
Morphology, hypertrophy, size, and systolic function of right ventricle |
Morphology and size of right atrium |
Morphology, size, and function of pulmonic valve, including the systolic orifice area, and the presence of valvular regurgitation |
Presence, size, morphology, and site of infundibular or subinfundibular obstruction |
Presence, size, morphology, and site of pulmonary artery(ies) obstruction |
Anatomy, size, and function of tricuspid valve |
Coexisting anomalies |
Following interventions, cardiac CT can be used to exclude or confirm recurrent obstruction. The major limitation of cardiac CT is its inability to measure pressure gradients across the stenotic lesions.
14.2 Pulmonary Atresia with Intact Ventricular Septum
14.2.1 Definition
Pulmonary atresia with intact ventricular septum is a rare congenital heart anomaly characterized by an atretic pulmonary valve, variable degrees of right ventricular and tricuspid valve hypoplasia, and coronary artery anomalies [23].
This anomaly accounts for 3 % of congenital heart defects with an incidence of 0.08 per 1,000 live births [24]. Despite the low prevalence, it is not an uncommon cause of cyanotic heart diseases in neonates.
14.2.2 Morphology
In this disorder, right ventricular morphology may vary. In its most mild form, there is a mildly hypoplastic chamber with a well-formed infundibulum and imperforate tricommissural pulmonary valve. As the severity progresses, a moderately hypoplastic chamber to a severely hypoplastic chamber with a narrowed or atretic infundibulum may be seen. Finally, a dysplastic pulmonary valve and ventriculocoronary artery connections may occur [25]. See Figs. 14.6 and 14.7. In addition, the tricuspid valve is dysplastic and demonstrates a spectrum of abnormalities, ranging from severe stenosis to severe regurgitation [26]
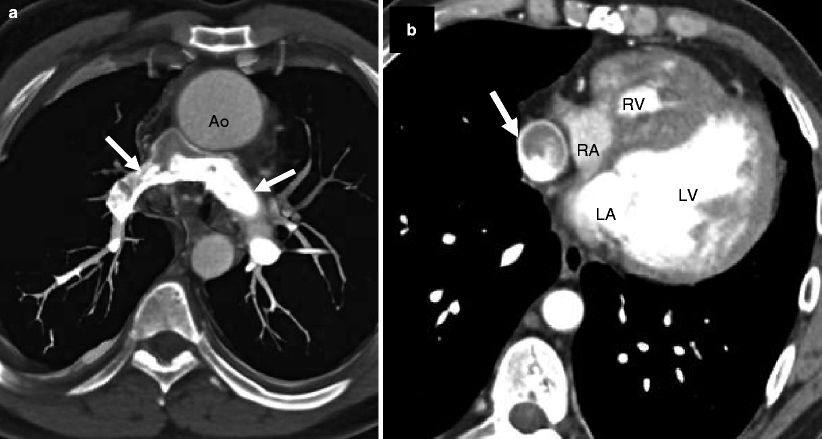
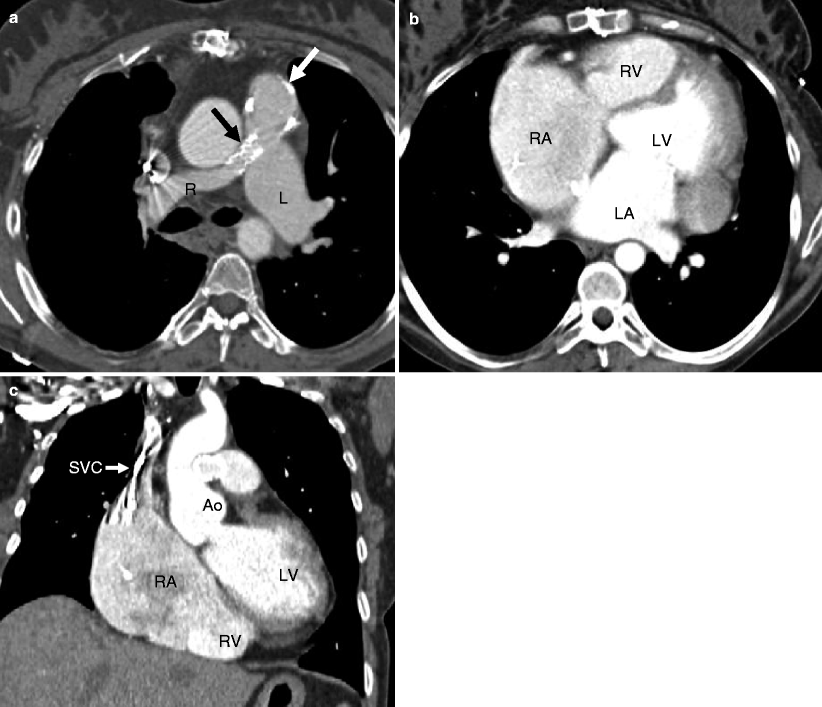

Fig. 14.6
Pulmonary atresia with intact ventricular septum in a 32-year-old man with ventriculocoronary connections who underwent a Fontan procedure. Panel (a) is an axial computed tomogram (CT) showing the absence of the main pulmonary artery and slightly diminutive right and left pulmonary arteries (arrows). Panel (b) is an axial CT at a more caudal plane demonstrating a severely hypoplastic right ventricle (RV) and the Fontan conduit (arrow). For comparison, the left ventricle (LV) is normal in size. Ao aorta, LA left atrium, RA right atrium

Fig. 14.7
Pulmonary atresia with intact ventricular septum. This is an example of a 40-year-old woman who had undergone right ventricular outflow tract reconstruction, patch enlargement of the main pulmonary artery, and stenting of the right pulmonary artery as a child. Panel (a) an axial scan showing a calcified pulmonary artery patch graft (white arrow) and a stent (black arrow) in a small proximal right pulmonary artery (R). The left pulmonary artery (L) is normal size. Panels (b) (coronal section) and c (axial slice) show mild to moderate right ventricle (RV) hypoplasia, normal left ventricle (LV) size, and right atrial (RA) enlargement. The streaks in the superior vena cava (SVC) are flow artifacts. LA left atrium, Ao aorta
The diminutive, hypertrophic right ventricle often exhibits diffuse fibroelastosis [27, 28]. Ebstein anomaly coexists in 10 % of cases [26]. Furthermore, there is an obligatory right-to-left atrial shunt (via a patent foramen ovale or atrial septal defect). Pulmonary blood flow usually depends on a patent ductus arteriosus. Aortopulmonary collaterals originating from the thoracic aorta are rarely seen. The main, right, and left pulmonary arteries are usually small but rarely are atretic (Fig. 14.8).
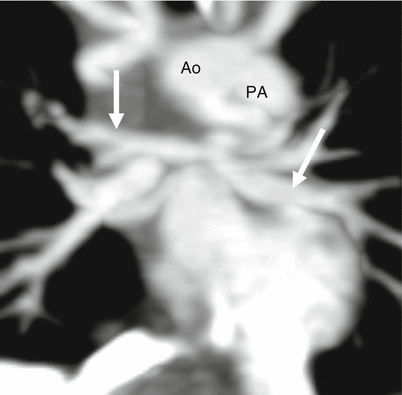

Fig. 14.8
Pulmonary atresia with intact ventricular septum. This coronal reconstruction shows small main (PA) as well as small right and left pulmonary arteries (arrows). Ao aorta
The right atrium is usually enlarged. Left-sided volume overload from right-to-left atrial shunting can result in left atrial and left ventricular enlargement. Distortion of the interventricular septum and right ventricular hypertrophy may cause left ventricular outflow tract obstruction. Right ventricle-to-coronary artery fistulas (sinusoids), with or without stenosis, atresia, or ectasia, occur in nearly 50 % of patients at birth [25].
14.2.3 Clinical Findings
Patients present in the neonatal period with cyanosis. Early survival depends on ductal patency until surgical repair can be performed to establish adequate pulmonary blood flow. Patients who have not undergone early surgical repair and have a patent ductus arteriosus may present as adults with dyspnea and mild central cyanosis [29, 30]. Surgically treated patients can present later in life with complications related to surgery [31, 32] and/or with sudden death, angina, and/or arrhythmias related to poor myocardial perfusion [26, 33].
14.2.4 Surgical Repairs
A palliative systemic-to-pulmonary artery shunt is done early in the neonatal period to establish an adequate source of pulmonary blood flow. The choice of subsequent definitive repair depends on the size and morphology of the right ventricle and tricuspid valve as well as the presence and extent of ventriculocoronary artery sinusoids (right-ventricular-dependent coronary circulation). The surgical options include biventricular, one-and-a-half-ventricle, and univentricular approaches [34]. Patients with the Ebstein-like variant may require tricuspid valve repair or conversion to tricuspid atresia physiology [35]. Patients with severe right ventricle-to-coronary artery sinusoids may need cardiac transplantation [36].
The biventricular repair approach is utilized if there is mild right ventricular and tricuspid valve hypoplasia without ventriculocoronary connections and without right ventricular outflow tract obstruction; a patch graft enlargement of the outflow tract and a surgical valvotomy (or, more recently, transcatheter perforation of the pulmonary valve) are performed. Controversy exists regarding the efficacy and safety of transcatheter valvotomy [36]. Procedure-related complications are not uncommon with this repair, with the mortality reaching 6 %. In addition, a substantial proportion of patients who undergo catheter valvotomy will need additional systemic-to-pulmonary shunts [37]. Pulmonary valvotomy is not performed in patients with right-ventricular-dependent coronary circulation since it can cause flow reversal in the ventriculocoronary sinusoids (coronary steal-like phenomenon), leading to myocardial ischemic injury.
The one-and-a-half-ventricle approach is applied when there is moderate to severe right ventricular and tricuspid valve hypoplasia without ventriculocoronary connections; right ventricular outflow tract reconstruction and surgical or percutaneous valvotomy with bidirectional cavopulmonary anastomosis is performed [36]. An atrial septostomy may be needed to decompress the right atrium [38].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


