Fig. 13.1
Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) risk score. (a) The chart used to calculate the integer risk score for each patient. (b) Distribution of the integer risk score and association with the risk of dying within 3 years (Adapted from Pocock et al. [2])
Patient evaluation begins with history and physical examination. Investigations include a chest X-ray and routine blood tests. Exercise tolerance is determined by cardiopulmonary exercise testing and quantified by measurements of maximal oxygen consumption (VO2). The patient is screened for viral disease and the presence of reactive antibodies to human leukocyte antigen (HLA). Right heart catheterisation and coronary angiography are performed before the patient is placed on the waiting list. This allows determination of pulmonary artery (PA) pressure and the presence of coronary artery disease. Further tests include thyroid function, blood glucose, creatinine clearance, electrocardiography, echocardiography and pulmonary function tests. Final selection of patients is based on subjective and objective criteria based on the patient’s projected survival without transplantation.
Contraindications to heart transplantation include advanced age. Comorbidity increases with age and is associated with reduced post-transplant survival. Elevated pulmonary vascular resistance (PVR) is an absolute contraindication to orthotopic heart transplantation. If the PVR is fixed above six Wood units and the pressure drop across the pulmonary circulation (transpulmonary gradient: mean PA pressure − pulmonary capillary wedge pressure) is greater than 15 mmHg without evidence of reversibility with vasodilators, the patient is not suitable for transplantation. Elevated PVR predicts post-transplant right heart failure, which can be fatal. Patients with elevated PVR can be considered for support with a left ventricular assist device (LVAD). This manoeuvre reduces left ventricular filling pressures and pulmonary venous hypertension, and a period of LVAD support in these patients may reduce PVR to levels considered acceptable for heart transplantation [3]. Patients with irreversible PAH may be considered for heart-lung transplantation. Diabetes is a contraindication in the presence of end-organ damage such as retinopathy, neuropathy or nephropathy. Active infection, hepatic dysfunction, malignancy, advanced lung disease and peripheral vascular disease are largely considered to be contraindications to transplantation. In very selected patients, simultaneous heart-kidney transplants can and have been successfully done. The patients’ psychosocial condition is also important, as they are required to adhere to a strict regimen of medical therapy and regular clinical follow-up.
13.3 Post-transplant Right Heart Failure
Right heart failure after heart transplantation remains a common and difficult problem to manage. It is a major contributor to overall mortality and morbidity in heart transplant patients – accounting for 50 % of all cardiac complications and 19 % of early deaths [4]. Its pathophysiology is complex and multifactorial, and the link with PAH has been known since the early days of heart transplantation [5]. The relationship between elevated PVR in the recipient and subsequent postoperative RV failure – and increased risk of death – has been recognised since the early 1970s [6, 7]. In addition to an increase in mortality, the occurrence of RV failure is also associated with an increase in all other complications [8].
13.4 Pathophysiology
Right ventricular failure following heart transplantation is most commonly due to the exposure of the donor heart, which is not adapted to high PVR, to an increased afterload in the recipient. Varying degrees of PAH are present in patients with chronic heart failure. Left ventricular failure results in an elevated left ventricular end-diastolic pressure and left atrial pressure and an increase of back pressure onto the pulmonary vasculature. This is further complicated by reactive pulmonary vascular vasoconstriction and an increase in PVR. The latter, in the longer term, may become irreversible [9]. The degree of PVR can be aggravated in the perioperative period with use of cardiopulmonary bypass, blood products and vasoactive drugs. Other risk factors include distant organ procurement and prolonged ischaemic time, poor myocardial preservation, warm ischaemia during surgery and reperfusion injury (Table 13.1).
Table 13.1
Factors contributing to RV failure after heart transplantation
Pre-existing or acquired pulmonary arterial hypertension |
Marginal organ preservation |
Long ischaemic time |
Significant (>20 %) donor-recipient mismatch |
Acute allograft rejection |
As described in the pathophysiology chapter, faced with all these challenges, the thin-walled RV has limited contractile reserve to cope with an acute increase in afterload. As the RV end-diastolic volume increases, wall tension increases and the RV becomes ischaemic. The interventricular septum shifts to the left, and as both the LV and RV are interdependent, this will result in a small, underfilled LV, leading to low cardiac output syndrome and cardiogenic shock (Fig. 13.2) [9, 10].
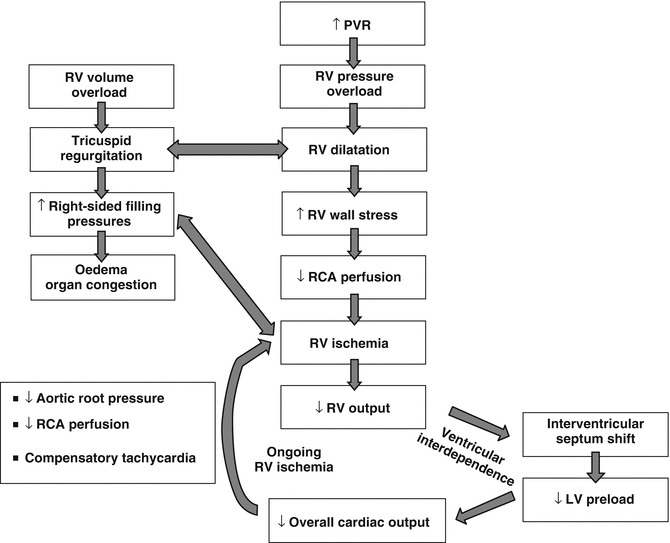

Fig. 13.2
Pathophysiology of right ventricular failure
13.5 Management
Effective therapy for RV failure remains very challenging. The primary goal is to prevent a low cardiac output syndrome. The principles include:
Maximising coronary perfusion – maintenance of adequate mean arterial pressure
Optimising myocardial oxygen delivery and limiting ventricular oxygen consumption
Optimising RV preload to a potentially distended, impaired and/or ischaemic RV
Reducing RV afterload by reducing PVR
Increasing contractility
Correction of arrhythmias and atrioventricular conduction to maintain adequate stroke volume of the right and left ventricles
Strategies for the optimisation of the individual parameters often overlap (Table 13.2). Management is instituted under strict conditions with adequate monitoring using invasive arterial and central venous pressure recordings, Swan-Ganz catheter and echocardiography. Preload optimisation is carried out with great care, while carefully monitoring right-sided filling pressures and cardiac output. Volume therapy should cease if it is associated with a large increase in right atrial pressure without an increase in cardiac output. Inotropic support is commenced to improve myocardial contractility. In most centres, catecholamines are used as first line. Epinephrine is widely used and improves cardiac output and perfusion pressure. However, it increases myocardial oxygen consumption and can be arrhythmogenic. At high doses, vasoconstriction of the systemic and pulmonary vasculature may be detrimental. In contrast, isoproterenol, a nonselective beta agonist, has a positive inotropic and chronotropic action in addition to being a pulmonary and peripheral vasodilator.
Table 13.2
Management strategy of acute RV failure after heart transplantation
Preservation of coronary perfusion |
Adequate mean arterial pressure |
Use of vasoconstrictors (noradrenaline) in combination with inodilators |
Optimisation of RV preload |
Judicious fluid administration (monitoring right-sided filling pressures and cardiac output) |
Use of diuretics and/or renal replacement therapy in distended, volume-overload RV |
Reduction in RV afterload |
Inodilators (isoproterenol, phosphodiesterase III inhibitors, levosimendan) |
Inhaled nitric oxide |
Inhaled prostacyclins |
Administration of 100 % oxygen and optimal PEEP on mechanical ventilation |
Avoidance of hypercapnia and acidosis
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|