The short lifespan of right ventricle–to–pulmonary artery (RV-PA) conduits used in repairs of complex congenital heart defects makes future surgical replacement inevitable. Percutaneous pulmonary valve implantation (PPVI) now offers an attractive alternative to surgery in some patients. The objectives of this study were to examine the pattern of conduit reoperations, the factors affecting conduit longevity, and to discuss the role of PPVI in these patients. Forty-nine patients (mean age 27 ± 8 years) with pulmonary atresia or pulmonary stenosis with tetralogy of Fallot who underwent surgery for RV-PA conduits from September 1974 to October 2011 were reviewed. A total of 106 RV-PA conduits were implanted, 57 of which were replacements. Second, third, and fourth conduits were required during the follow-up period in 39, 16, and 2 patients, respectively. Freedom from reoperation at 10 years for the first, second, and third conduits was 50%, 74%, and 86%, respectively. Significant independent predictors of shorter conduit longevity included smaller conduit and conduit type (homograft and other vs Dacron). Furthermore, a significant proportion (32 of 57 [56%]) of conduit replacements took place from ages 9 to 18 years. There were 37 adults whose current existing conduits had not yet failed, with 73% (27 of 37) potentially suitable in the future for PPVI on the basis of conduit size of 16 to 27 mm. In conclusion, multiple RV-PA conduit revisions were required in patients who survived to adulthood, with many replacements taking place during adolescence. Most conduits in this adult cohort met size criteria for PPVI, thereby offering these patients a potential alternative to surgical intervention for conduit failure.
Rastelli et al pioneered a method for the repair of complex congenital heart disease (CHD) when they implanted a right ventricle–to–pulmonary artery (RV-PA) pericardial tube conduit for pulmonary atresia in 1964. RV-PA conduits continue to play an important role in the surgical repair of CHD but remain plagued by high failure rates, with only half of patients free from reoperation 10 years after initial conduit implantation. In many cases, conduit failure is related to somatic outgrowth in young patients and progressive conduit calcification resulting in stenosis and/or regurgitation. Chronic right ventricular pressure and/or volume overload imposed by conduit failure can lead to right ventricular dilatation and dysfunction. This is associated with reduced exercise intolerance and a propensity for arrhythmias or sudden cardiac death. In children who survive to adulthood, conduit failure is inevitable, and the likelihood of facing another reoperation is high. However, the recent development of percutaneous pulmonary valve implantation (PPVI) offers a safe and viable surgical alternative in select patients who develop conduit stenosis and/or regurgitation. At present, PPVI is not suitable for all patients, and the optimal timing remains uncertain. The purpose of this study was twofold: (1) to determine the factors affecting RV-PA conduit longevity in an adult population with tetralogy of Fallot or pulmonary atresia, and (2) to examine the patterns of conduit reoperation. Our findings will be used to guide a discussion regarding the role of PPVI.
Methods
Forty-nine adult patients with RV-PA conduits implanted for pulmonary atresia or pulmonary stenosis in the setting of tetralogy of Fallot from September 1974 to October 2011 were identified from the Pacific Adult Congenital Heart Clinic database at St. Paul’s Hospital (Vancouver, British Columbia, Canada) and were all included in this study. All patients were transferred to the Pacific Adult Congenital Heart Clinic for continued care in adulthood, allowing complete follow-up of the study population. Patient charts were reviewed retrospectively and data were collected, including CHD diagnosis and operative procedures before first conduit implantation. For each surgery, patient age, date, conduit size, conduit type, and postoperative ratio of right ventricular systolic pressure to left ventricular systolic pressure (right ventricular pressure as a percentage of left ventricular pressure measured in the ascending aorta in the absence of left ventricular outflow tract obstruction) were obtained. Institutional review board approval was obtained.
Echocardiographic and cardiac catheterization records within 12 months before conduit intervention were reviewed to determine the main indication for conduit failure, which was classified as severe conduit stenosis, regurgitation, stenosis and regurgitation, or endocarditis. Peak gradients across conduit stenoses and the severity of conduit regurgitation were based on echocardiographic and cardiac catheterization records. Conduit regurgitation was classified qualitatively from echocardiographic reports as none, mild, moderate, or severe. Severe conduit stenosis or regurgitation was deemed to require intervention in the presence of symptoms and/or progressive right ventricular dilatation or dysfunction.
Details of all percutaneous cardiac procedures were reviewed. Nonconduit percutaneous interventions (angioplasty, stenting) were those performed for pulmonary artery stenosis. Direct conduit percutaneous interventions (angioplasty, stenting, PPVI) were those performed in lieu of or to delay conduit replacement in patients whose conduits had reached end of life. PPVI can be performed with the Melody transcatheter heart valve (THV; Medtronic, Inc., Minneapolis, Minnesota) and the SAPIEN THV (Edwards Lifesciences, Irvine, California). Edwards SAPIEN and SAPIEN XT THVs are suitable for conduit diameters of 18 to 27 mm. In comparison, Melody THVs are suitable for conduit diameters of 16 to 22 mm. Therefore, we considered patients potential candidates for PPVI if their conduit diameters were between 16 and 27 mm inclusive.
Patient, conduit, and procedural characteristics are expressed as mean ± SD or as median (interquartile range [IQR]) for continuous variables with normal or non-normal distributions, respectively, and as frequencies and proportions for categorical variables. Different statistical tests were performed for comparisons of conduit-specific variables across conduit groups (first, second, third, and fourth conduit): mixed-model analysis of variance for conduit diameter and age at surgery with Tukey adjusted pairwise comparison, the log-rank test for Kaplan-Meier estimates of freedom from conduit replacement with Sidak adjustment for pairwise comparison, and the chi-square test for overall comparison of conduit type. Potential factors affecting conduit longevity were identified on the basis of published research and clinical evidence and assessed univariately using proportional-hazards regression for recurrent events. All identified factors were evaluated in a multivariate proportional-hazards model of recurrent events for their independent effects on conduit longevity. Factors that had neither significant independent effects nor univariate associations were removed from the final multivariate model. The proportional-hazards assumption was tested on the basis of the interaction term with log-transformed time. No violation of the proportionality assumption was identified. A p value 0.05 was used as the significance level for statistical tests. All analysis was performed using SAS version 9.3 (SAS Institute Inc., Cary, North Carolina).
Results
The patient population consisted of 31 male (63%) and 18 female (37%) patients, with a mean age of 27 ± 8 years. There were 16 patients (33%) with pulmonary atresia, with all but 4 patients having ventricular septal defects. The other 33 patients (67%) had pulmonary stenosis in association with tetralogy of Fallot. The median age at the time of first conduit implantation was 4 years (IQR 2 to 10). The median follow-up time since first conduit implantation was 19 years (IQR 16 to 23). The associated cardiac procedures performed before first conduit implantation are listed in Table 1 . There were a total of 4 deaths in the cohort. One patient (age 22 years) died in the postoperative period from cardiac arrest and multiple organ failure after the placement of a third conduit. Causes of late death in the other 3 patients were sepsis with multiple organ failure (age 57 years), cardiac arrest (age 20 years), and suicide (age 20 years).
| Procedure | Number of Procedures | Number of Patients |
|---|---|---|
| Blalock-Taussig shunt | 46 | 31 |
| Pulmonary artery repair/enlargement | 15 | 12 |
| Right ventricular outflow tract reconstruction | 13 ∗ | 11 |
| Blalock-Taussig shunt ligation | 8 | 6 |
| Ventricular septal defect closure | 7 † | 6 |
| Pulmonary valvotomy | 5 | 5 |
| Waterston shunt | 4 | 4 |
| Atrial septostomy | 3 | 3 |
| Atrial septal defect closure | 3 | 3 |
| Aortopulmonary collateral vessels ligated/embolized | 3 | 3 |
| Pulmonary artery banding | 2 | 2 |
| Tricuspid valve commissurotomy | 2 | 2 |
| No previous cardiac procedures | 10 | 10 |
∗ One patient required 3 repairs.
Overall, 106 RV-PA conduits were implanted in 49 patients, and 57 of these conduits were replacements in 39 patients. Medians of Kaplan-Meier estimates for times to replacement for first, second, and third conduits are listed in Table 2 . A significant proportion (32 of 57 [56%]) of conduit replacements occurred from ages 9 to 18 years ( Figure 1 ). Many patients required second (n = 39) or third (n = 16) conduits, but only 2 received fourth conduits.
| Characteristic | Conduit 1 | Conduit 2 | Conduit 3 | Conduit 4 | p Value |
|---|---|---|---|---|---|
| Number of patients | 49 | 39 | 16 | 2 | |
| Age at conduit surgery (yrs) | 7.6 ± 8.7 | 14.7 ± 8.5 | 20.2 ± 10.3 | 13.0 ± 1.4 | <0.001 |
| Conduit size (mm) ∗ | 18.7 ± 4.5 | 24.5 ± 3.4 | 25.2 ± 3.1 | 28.5 ± 2.1 | <0.001 |
| Conduit type | 0.043 | ||||
| Dacron | 6 (12%) | 13 (33%) | 8 (50%) | 1 (50%) | |
| Homograft | 36 (74%) | 22 (56%) | 7 (44%) | 1 (50%) | |
| Other | 7 (14%) | 4 (10%) | 1 (6%) | 0 (0%) | |
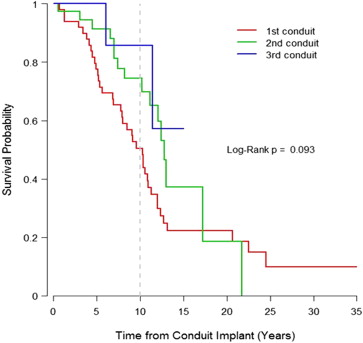
| Conduit longevity (yrs) | 10.3 (5.2–12.7) | 12.8 (8.3–17.2) | >15.0 (11.4–>15.0) | — | 0.093 † |
∗ Number of missing conduit sizes (conduit 1 = 8, conduit 2 = 2).
† Log-rank test for Kaplan-Meier curves comparison among conduits 1, 2, and 3; for conduit 3, the median and 75th percentile of longevity were greater than the maximum follow-up time of 15 years.
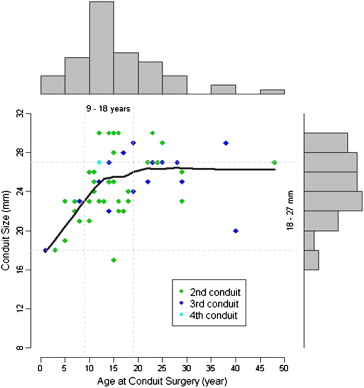
Indications for conduit replacement included severe conduit stenosis (38 of 57 [67%]) or regurgitation (7 of 57 [12%]), combined stenosis with regurgitation (10 of 57 [18%]), and endocarditis (2 of 57 [3%]). Severe conduit stenosis was secondary to calcific and degenerative changes. The second conduit implanted was significantly larger than the first conduit (25 ± 3 vs 19 ± 5 mm, p <0.001). There was no significant difference in size between second and third conduits (25 ± 3 mm, p = 0.37) or between third and fourth conduits (29 ± 2 mm, p = 0.27). Ten-year freedom from reoperation after the first, second, and third conduits was 51% (95% confidence interval [CI] 38% to 67%), 75% (95% CI 60% to 92%), and 86% (95% CI 63% to 100%), respectively ( Figure 2 ). Although there was a trend to significance, these differences did not reach statistical significance (p = 0.09).
Overall, conduit implantation after versus during early childhood was associated with better conduit longevity. Taking all conduits into consideration, 10-year freedom from reoperation on the basis of age of implantation was 47% (95% CI 34% to 64%) for ages 0 to 8 years, 83% (95% CI 72% to 95%) for ages 9 to 18 years, and 44% (95% CI 16% to 100%) for adulthood ( Figure 3 ). Freedom from reoperation was significantly better for conduits implanted from ages 9 to 18 years versus 0 to 8 years (p = 0.008), but the difference between conduits implanted from 9 to 18 years versus adulthood did not reach statistical significance (p = 0.07). Note that the sample size for adulthood implantation was small (n = 19) compared with the groups aged 0 to 8 years (n = 43) and 9 to 18 years (n = 44).
Various risk factors affecting conduit longevity are listed in Table 3 . On the basis of multivariate proportional-hazards model analysis, smaller conduit diameter and conduit types “other” and homograft (compared with Dacron graft) were significant predictors of conduit failure. However, gender, percutaneous nonconduit procedures, and right ventricular/left ventricular systolic pressure ratios measured in the immediate postoperative period after conduit replacement were not predictive of conduit longevity. Because conduit diameter was highly correlated with age at implantation (Pearson’s correlation coefficient r = 0.62, p <0.001), age was also not an independent predictor of conduit longevity in the multivariate model incorporating conduit size.
| Factor | Comparison | Univariate | Multivariate | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Age | Per 5-yr increase | 0.75 (0.48–1.18) | 0.217 | ||
| Gender | Male vs female | 1.55 (0.87–2.77) | 0.140 | ||
| Diagnosis | TOF vs pulmonary atresia | 1.35 (0.74–2.46) | 0.326 | ||
| Conduit size | Per 1-mm decrease | 1.15 (1.05–1.26) | 0.004 | 1.14 (1.04–1.24) | 0.006 |
| Conduit type | Dacron | 1 (reference) | 1 (reference) | ||
| Homograft | 2.70 (1.12–6.48) | 0.027 | 2.51 (1.00–6.32) | 0.050 | |
| Other | 5.80 (2.00–16.87) | 0.001 | 4.61 (1.23–17.31) | 0.024 | |
| Percutaneous nonconduit procedure | Yes vs no | 1.03 (0.43–2.46) | 0.941 | ||
| RV/LV systolic pressure ratio | Per unit increase | 1.53 (0.24–9.60) | 0.651 | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


