11 Right Heart Anomalies
Basic Principles
Right Ventricle
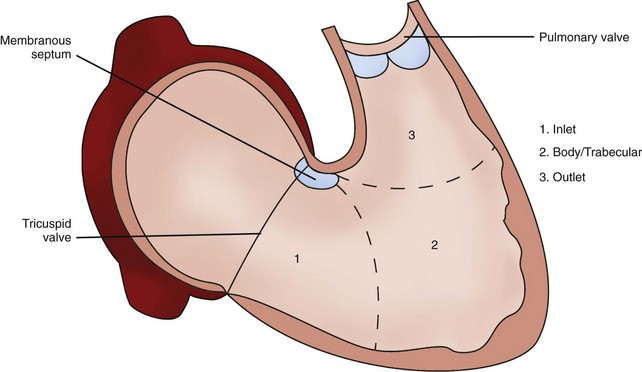
• There are three portions of a normally formed morphologic right ventricle (RV): inlet (supports the tricuspid valve [TV]), body (trabecular portion), and outlet (infundibulum or conal region).1
• The tripartite RV is loosely crescent shaped rather than conical shaped like the left ventricle (LV).
• Coarse muscular trabeculations and numerous small papillary muscles with attachments to both the septal and free walls further help to define a normal morphologic RV2 (Fig. 11-1).
Ebstein’s Anomaly
Background
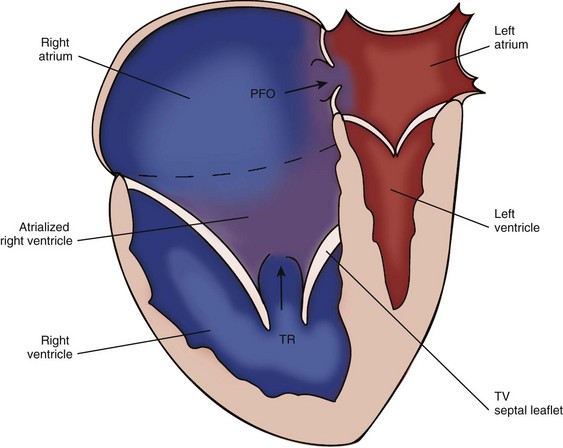
• Ebstein’s anomaly (or malformation) is characterized by an increased downward (apical) displacement of the TV annulus caused by failure of delamination of the posterior and septal leaflets.3
• There is subsequent variable thinning of the wall of the “atrialized” RV (aRV) along with redundancy and tethering of the anterior leaflet of the TV.
• The clinical manifestations of Ebstein’s anomaly depend largely on the degree of severity of the TV displacement and the resultant physiologic effects.
• Right ventricular function becomes impaired and the abnormal TV becomes regurgitant, resulting in decreased forward flow through the pulmV.
• The combined right atrium (RA) and aRV becomes dilated with resultant right-to-left shunt across the interatrial septum (IAS).
• Clinically, infants with severe Ebstein’s anomaly will present with extreme cyanosis, cardiomegaly, and heart failure.
• Most patients with Ebstein’s anomaly will have other associated cardiac lesions. The most common associated lesion is an atrial septal communication, usually a patent foramen ovale (PFO), or secundum atrial septal defect (ASD). Other associated lesions include pulmV stenosis or atresia, ventricular septal defect (VSD), tetralogy of Fallot (TOF), left ventricular noncompaction (LVN), coarctation of the aorta (Ao), as well as other left-sided heart lesions. In congenitally corrected transposition of the great arteries (cc-TGA), the systemic left-sided TV may have some degree of Ebstein’s anomaly3–5 (Fig. 11-2).
Overview of Echocardiographic Approach
• The majority of clinically important information will be acquired with transthoracic two-dimensional (2D) imaging. The remaining physiologic data can be obtained with spectral and color Doppler.
• The goal of imaging is to define the anatomy associated with Ebstein’s anomaly and to describe the severity of the lesion.
Anatomic Imaging
Acquisition
• Transthoracic echo (TTE) in the neonate is the diagnostic gold standard and defines the severity of Ebstein’s anomaly.
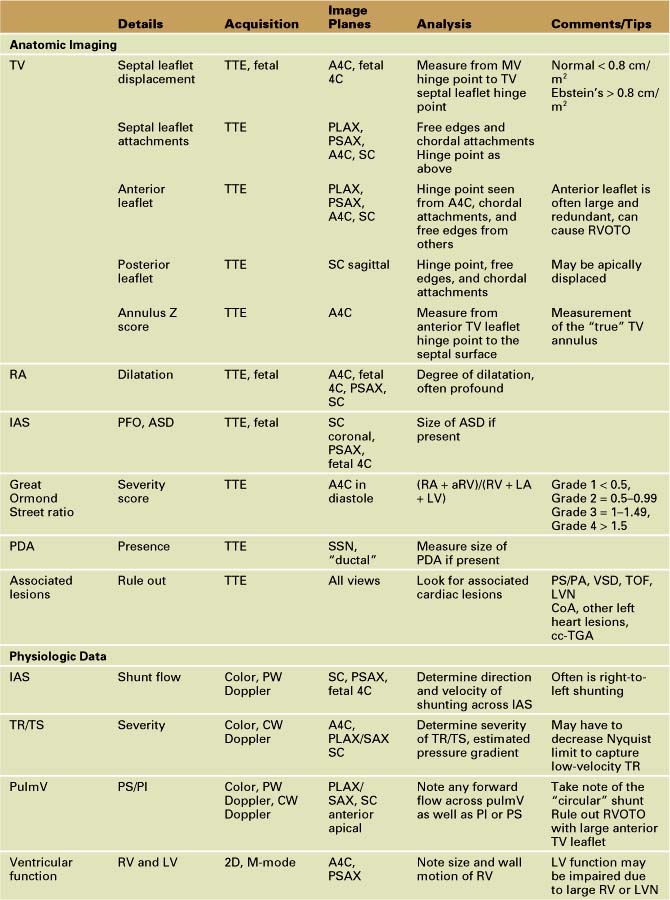
• Parasternal long axis and short axis (PLAX and PSAX), subcostal long axis and short axis (SCLAX and SCSAX), and apical four-chamber (A4C) views are the most useful imaging planes to visualize the RA, IAS, TV, RV, and pulmV.
• Transesophageal echo (TEE) is used intraoperatively to assess the adequacy of the surgical repair.
• In the adult patient with Ebstein’s anomaly, TTE is usually adequate to visualize the right heart. However, TEE may be required if the TV leaflets are difficult to visualize with TTE.
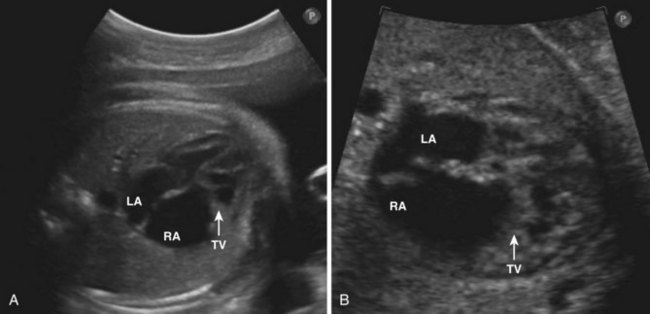
• In fetal echo, the TV abnormality is best seen in the 4C view. The cardiothoracic ratio is also useful to demonstrate the extreme cardiomegaly seen with Ebstein’s anomaly (Fig. 11-3).
Analysis
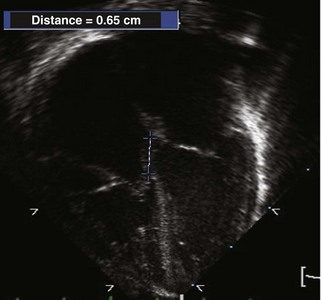
• The septal TV leaflet (and the posterior/inferior leaflet in severe forms) is apically displaced relative to the anterior leaflet of the MV. A displacement more than 0.8 cm/m2 is diagnostic of Ebstein’s anomaly. The A4C view best demonstrates the displacement and allows for measurement6 (Fig. 11-4).
• The profound right atrial dilation characteristic of Ebstein’s anomaly may best be seen in the A4C view, parasternal short axis view, and the subcostal view.
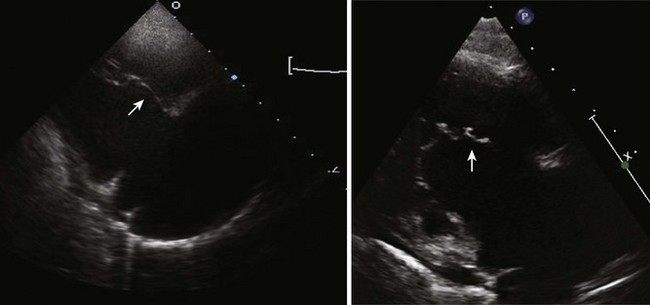
• Define the anatomy of the TV anterior, septal, and posterior leaflets (including attachments, tethering, dysplasia, and redundancy). The TV is best visualized from parasternal long axis and short axis, A4C, and subcostal views.5 Using an anatomic classification described by Carpentier et al.,7 a type A, B, C, or D category may be designated (Table 11-2). This classification can be helpful to the surgeon when considering whether to repair the TV6 (Fig. 11-5).
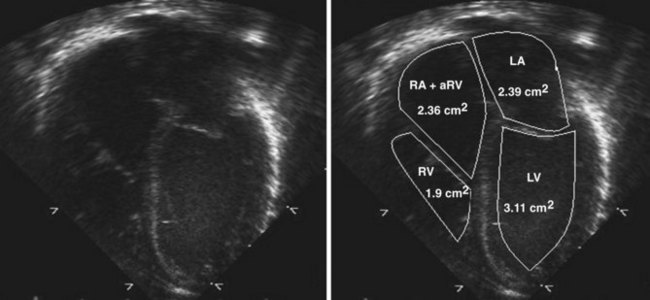
• The echocardiographic assessment of severity can be measured using the Great Ormond Street (GOS) ratio described by Celermajer et al.9 The ratio is obtained from the A4C view in end-diastole. It is the ratio of the area of the RA plus the area of the aRV compared with the area of the functional RV plus the left atrial and left ventricular areas (RA + aRV)/(RV + LA + LV). The increasing grade of severity is grade 1, ratio less than 0.5; grade 2, ratio 0.5 to 0.99; grade 3, ratio 1 to 1.49; grade 4, ratio greater than 1.5. Grades 3 and 4 have an increased risk of mortality9 (Fig. 11-6).
• Other important anatomic features to define include the normalized dimension of the TV annulus via Z score (usually at the level of the anterior leaflet proximal hinge points, where the true tricuspid annulus is located), the size of the ASD (if present), the presence or absence of a patent ductus arteriosus (PDA), and other associated cardiac anomalies (discussed previously).10
TABLE 11-2 ANATOMIC CLASSIFICATION
| Type A | Septal and posterior leaflet adherance without functional RV restriction of volume |
| Type B | Atrialized RV with normal anterior leaflet hinge point |
| Type C | Anterior leaflet stenosis |
| Type D | RV entirely atrialized except for a small infundibulum |
Data from Carpentier A, Chauvaud S, Mace L, et al. A new reconstructed operation for Ebstein’s anomaly of the tricuspid valve. J Thorac Cardiovasc Surg. 2006;132:1285–1290.
Pitfalls
• It is sometimes difficult to distinguish isolated TV dysplasia from true Ebstein’s anomaly. In TV dysplasia, the functional right ventricular chamber is usually larger and the TV orifice is directed to the apex of the RV. In Ebstein’s anomaly, the functional RV is usually smaller and the TV orifice is directed to the right ventricular outflow tract (RVOT).
Physiologic Data
Acquisition
• After a thorough description of the anatomy of Ebstein’s anomaly has been achieved, the physiologic characteristics of the lesion should be delineated.
• The use of pulsed wave (PW) Doppler, continuous wave (CW) Doppler, and color Doppler is of paramount importance to define the direction of blood flow through the heart: either forward flow through the RV and out the pulmV or reverse flow across the IAS to the LV, out the aortic valve (AV) and then across the PDA.
Analysis
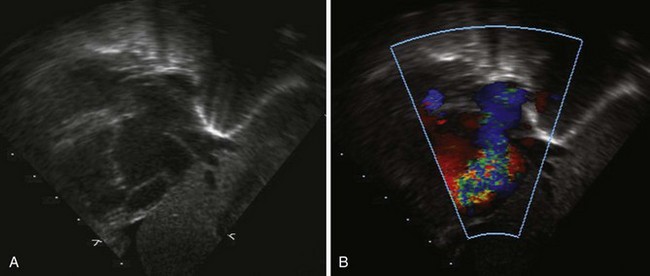
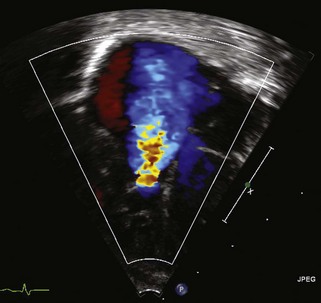
• Focusing on the IAS from either the subcostal or parasternal view, use color Doppler along with PW Doppler to demonstrate the direction and velocity of shunting across the ASD. There is usually right-to-left atrial-level shunting in hemodynamically significant Ebstein’s anomaly (Fig. 11-7).
• From the A4C view as well as the parasternal and subcostal views, use color Doppler to demonstrate the severity of TV regurgitation (TR) and/or stenosis (TS). Use CW Doppler of the tricuspid regurgitant jet to estimate functional right ventricular pressure (Fig. 11-8).
• Investigate the pulmV with color Doppler, PW Doppler, and CW Doppler for forward flow, insufficiency, and stenosis from the parasternal short axis and long axis views as well as the subcostal views. This may be seen from an anteriorly directed apical view as well. Take special care to note pulmonary insufficiency (PI) in the setting of tricuspid regurgitation (TR), right-to-left atrial-level shunting, and left-to-right ductal shunting (the so-called circular shunt). Also pay special attention to the RVOT, as a large, redundant anterior leaflet of the TV can cause RV outflow tract obstruction (RVOTO). Some patients may exhibit “functional” pulmonary stenosis (PS) in which the valve leaflets fail to open in the setting of reduced right ventricular output and accompanying elevated pulmonary artery pressure (PAP) of the newborn. Pulmonary regurgitation can be a tip-off that the pulmonary valve will in fact eventually open when the PAP incrementally decreases.
• Right and left ventricular function should be evaluated. Pay attention to the size and wall motion of the RV. Measure left ventricular shortening fraction with M-mode measurements from the parasternal short axis view at the level of the papillary muscles.
• TEE can be helpful in the setting of surgical repair of Ebstein’s anomaly (TV repair/replacement or RV exclusion). The preoperative assessment should be used to confirm the anatomy found on TTE and further define the TV attachments if needed. Postoperatively, it is important to assess the TV repair (residual TR or TS) or the right ventricular exclusion patch fenestration depending on the surgical repair or palliation. Evaluate the IAS, ventricular function, MV pulmV function, and the PDA (if applicable) as well.
Alternate Approaches
• Usually a complete diagnosis can be made based on echocardiographic data alone in Ebstein’s anomaly.
• On rare occasions, if there are anatomic questions unable to be answered by echo (TTE or TEE), other imaging modalities can be considered including MRI or cardiac catheterization. Catheterization is particularly important if hemodynamic data are required.
Key Points
• Ebstein’s anomaly is characterized by an increased apical displacement of the TV annulus caused by failure of delamination of the posterior and septal leaflets.
• The TV is often regurgitant into the atrialized portion of the RV, causing severe right atrial enlargement and right-to-left flow across an ASD.
• The clinical manifestations of Ebstein’s anomaly depend largely on the severity of the TV displacement and the resultant physiologic effects. Infants with severe Ebstein’s anomaly will present with extreme cyanosis, cardiomegaly, and heart failure.
• The echocardiogram is crucial for accurate diagnosis and prognostic interpretation of disease severity.
Pulmonary Atresia with Intact Ventricular Septum
Background
• Pulmonary atresia with intact ventricular septum (PA/IVS) is a rare and unique clinical entity in which the pulmV is atretic or imperforate and there is no interventricular communication.
• On the severe end of the spectrum, the pulmV is imperforate and the RV is severely hypoplastic. The TV is often dysplastic and hypoplastic as well. Due to the lack of egress of antegrade right ventricular blood flow, the right ventricular pressure is often suprasystemic. There is a higher incidence of coronary sinusoids from the RV in this scenario.
• RV-dependent coronary circulation (RVDCC) occurs in a small proportion of patients with PA/IVS. There may be stenosis that develops within the coronary artery system as well as ostial stenosis or atresia at the aortic cusp.5 As a result, the normal perfusion from oxygenated blood through the coronary arteries cannot take place. Instead, the perfusion to the myocardium becomes partially supplied by deoxygenated blood from the RV through coronary fistulous connections from persistent sinusoids. The presence of RVDCC has important clinical implications discussed further below.
• On the less severe end of the spectrum, patients can have pulmV atresia with a large or even dilated RV. These patients are less likely to have coronary sinusoids and more likely to have severe TR.
• Patients will initially require an intervention, either catheter based or surgical, to establish pulmonary blood flow (PBF). Depending on the severity of the lesion, including the presence or absence of RVDCC, the patient may eventually have a two-ventricle repair or a staged 1.5-ventricle or single-ventricle (SV) palliation.12–14
Overview of Echocardiographic Approach
TTE plays an integral role in defining the anatomy and clinical severity of patients with pulmonary atresia with IVS (Table 11-3).
• The goal of echo is to delineate the severity of right ventricular hypoplasia and related anatomy to help elucidate the physiology.
• In this lesion in particular, echo plays a complementary role to invasive diagnostic and interventional cardiac catheterization. One major complication of severe PA/IVS, RVDCC, cannot be identified with echo alone. The presence of RVDCC eliminates the possibility of a two-ventricle repair or a 1.5-ventricle palliation via opening the pulmV either surgically or interventionally and decompressing the RV.
Anatomic Imaging
Acquisition
• The initial 2D imaging should include defining the severity of right ventricular hypoplasia, measuring the TV Z score, evaluating the atretic pulmV and RVOT anatomy, identifying the main pulmonary artery (MPA) and branch pulmonary arteries, and identifying other associated lesions including a PFO and PDA.
Analysis
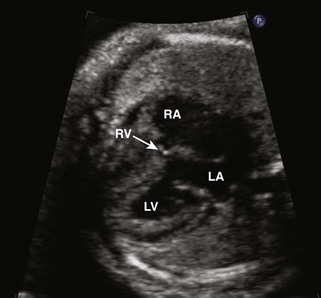
• As stated previously, the RV can be severely hypoplastic to severely dilated. The A4C view is a good place to start to get a sense of the size of the RV as well as the size of the inflow portion. By using multiple imaging plane sweeps (parasternal, subcostal), determine whether the RV is tripartite (inflow, body, and outflow) (Fig. 11-10).