21 Right Atrial Isomerism
I. CASE
A. Fetal echocardiography findings
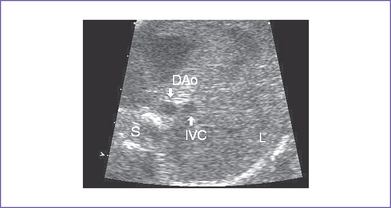
1. Imaging of the inferior vena cava (IVC) and aorta shows the two vessels running together on the left side of the spine (right atrial isomerism [RAI]) (Fig. 21-1).
2. The liver is midline, and the stomach is on the right and posterior.
3. The heart is in the left chest with a left aortic arch, a normal cardiac axis, and a normal size (cardiothoracic ratio = 0.34). The heart rate is 154 bpm.
4. The four-chamber view shows significant disproportion, with an unbalanced atrioventricular (AV) canal. There is a significantly large right-sided morphologic right ventricle (RV).
5. There is mild holosystolic common AV valve regurgitation.
6. The outflow assessment reveals normally related great arteries but with asymmetry (aorta–to–pulmonary artery diameter ratio = 1.1:0.6), and both arise from the RV.
7. No forward flow can be demonstrated by color or pulsed Doppler through the pulmonary outflow and main pulmonary artery. There are small confluent branch pulmonary arteries.
8. The aortic annulus is increased in size, and there is normal blood velocity by Doppler through it (1.2 m/s).
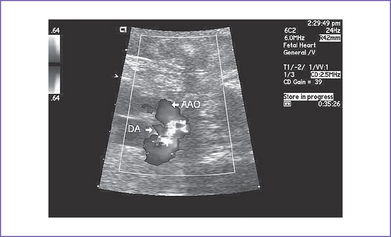
9. The ductal arch is tortuous and small and has retrograde flow (filling the pulmonary artery) (Fig. 21-2).
10. There is a large atrial septal defect (ASD) creating a common atrium, with a strand of muscle extending from anterior to posterior.
11. The pulmonary venous connection is to a posterior confluence and then to a descending vein coursing to the liver, where it is obstructed (abnormal venous flow pattern) before draining into the IVC.
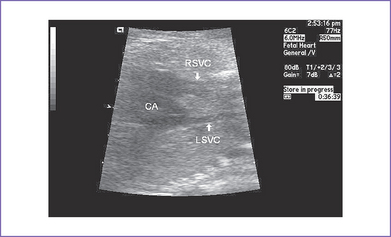
12. There are bilateral superior venae cavae (SVC) to the respective atria and no coronary sinus (Fig. 21-3).
13. The RV Tei index (myocardial performance index) is normal, with good ventricular function and no signs of heart failure.
D. Fetal management and counseling
a. The risk from amniocentesis is probably higher than the risk of abnormal karyotype in isomerism.
b. Aneuploidy is extremely rare and reportable in atrial isomerism.
2. Follow-up includes serial antenatal studies at 4- to 6-week intervals to monitor:
a. Heart size and ventricular function.
b. Evidence of hydrops fetalis, including pericardial effusion, ascites, and other features.
c. Progressive common AV valve regurgitation.
d. Size of the LV (when it is unbalanced at such an early age, it will remain so to delivery, and therefore reassessment of LV size is of no real value).
e. Size of the pulmonary arteries.
f. Pulmonary vein growth and flow patterns that could suggest progressive obstruction.
E. Delivery
1. In RAI with complex congenital heart disease, delivery should be at term or as close to term as possible in a tertiary care center given the risk of pulmonary stenosis and atresia and pulmonary vein obstruction.
2. With this ductus-dependent lesion, early initiation of prostaglandin E1 (PGE1) is mandatory. However, if obstruction of the pulmonary veins coexists, then increasing pulmonary perfusion can cause pulmonary edema.
F. Neonatal managment
a. PGE1 infusion should be started to keep the ductus open to increase pulmonary blood flow and raise arterial oxygen saturation to greater than 70%. If saturation does not increase, then repair of a total anomalous pulmonary venous connection (TAPVC) may be indicated.
b. Administration of oxygen can increase oxygen saturation by decreasing pulmonary vascular resistance (PVR) and by increasing blood flow.
c. Intubation and mechanical ventilation for progressive cyanosis might be needed.
d. With hypotension, volume and inotropic support are indicated to improve ventricular function.
e. Antibiotics: Asplenic patients should receive amoxicillin orally once per day at dose of 20 mg/kg body weight.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree