Keith-Wagener-Barker classification
Simplified (Wong-Mitchell) classification
Grade
Features
Grade
Features
1
Mild generalized retinal arteriolar narrowing
None
No detectable signs
2
Definite focal narrowing and arteriovenous nipping
Mild
Generalized arteriolar narrowing, focal arteriolar narrowing, arteriovenous nicking, opacity (“copper wiring”) of arteriolar wall or a combination of these signs
3
Signs of grade 2 retinopathy plus retinal hemorrhages, exudates, and cotton wool spots
Moderate
Retinal hemorrhages (blot, dot, or flame-shaped), microaneurysm, cotton wool spot, hard exudates, or a combination of these signs
4
Severe grade 3 retinopathy plus papilledema
Malignant
Signs of moderate retinopathy plus swelling of the optic disk
Subsequently, a simplified three-grade classification system according to the severity of the retinal signs was proposed by Wong and Mitchell [6] (Table 28.1, Fig. 28.1), based on the evidence that certain hypertensive retinopathy signs (e.g., arteriolar narrowing or arteriovenous nicking) are independently associated with cardiovascular (CV) risk. In a small study comprising 50 normal and 50 hypertensive fundi, respectively, inter- and intraobserver reliabilities of the simplified three-grade classification system and the traditional four-grade classification system introduced by Keith, Wagener and Barker were reported to be comparable [7].
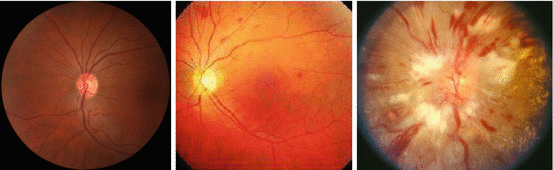

Fig. 28.1
Funduscopy (grade none, moderate, and malignant)
In the ESH/ESC guidelines, it is no longer recommended in general [8]. However, the retinal circulation offers the unique opportunity to visualize repeatedly the body’s microcirculation directly, noninvasively, and safely in vivo. Hence, in the last decade, new and more specific approaches were introduced to overcome these shortcomings and to detect reliable early changes of the retinal circulation.
28.2 Retinal Photographs/Funduscopy (Static)
In the last two decades, several large-scale, population-based studies assessing retinal photographs were conducted, including patients with and without hypertension. In (most of) these studies, standardized protocols of retinal photographs (45° nonstereoscopic color retinal photograph centered between the optic disk and the macula) were used to define specific signs of retinopathy, but not regarding a prespecified grading system. In part, retinal abnormalities were described based solely on qualitative parameters, such as tortuosity, arteriovenous crossing, caliber, and optic disk, but due to limited clinical usefulness, these data will not be reviewed. As further improvement, the imaging software “Interactive Vessels Analysis” (IVAN) (University of Wisconsin, Madison, WI, USA) has been established. This system conducts semiautomated measurement of retinal arterioles and venules, and hence its ratio (A/V ratio); however, it is not able to evaluate the retinal vascular wall directly.
Based on this approach, these studies have analyzed the relationship between retinal vascular alterations and their association with BP, TOD, and CV events which are (in part) summarized in Table 28.2.
Table 28.2
Large scale, population-based studies (in alphabetical order) assessing associations between retinal vascular caliber (based on retinal photography) and blood pressure, target organ damage, and cardiovascular risk (in chronological order)
Study | Country | Ethnicity | Year | Sample size | Retinal vascular | Finding |
|---|---|---|---|---|---|---|
Atherosclerosis Risk In Communities Study (ARIC) | USA | White, black | 1999 | 9,300 | A/V ratio | Past and current blood pressure [9] |
2001 | 10,358 | A/V ratio | Incident stroke [10] | |||
2002 | 9,648 | A/V ratio | Incident CHD, acute MI (only in women) [11] | |||
2004 | 5,628 | A/V ratio | Incident hypertension [12] | |||
(Only) black | 2008 | 1,439 | Generalized arteriolar narrowing | Left ventricular hypertrophy [13] | ||
A/V ratio | Left ventricular hypertrophy [13] | |||||
White, black | 2010 | 10,496 | Generalized arteriolar narrowing | Incident lacunar stroke [14] | ||
Generalized venular widening | Incident lacunar stroke [14] | |||||
Beaver Dam Eye Study (BDES) | USA | White | 2003 | 1,611 | A/V ratio | CV mortality (43–74 years) [15] |
2003 | 4,926 | Retinal arteriolar diameter | Current blood pressure [16] | |||
A/V ratio | Current blood pressure [16] | |||||
2004 | 2,451 | A/V ratio | Incident hypertension [17] | |||
2007 | 4,926 | Smaller arterioles | CHD death [18] | |||
Larger venules | CHD death [18] | |||||
Blue Mountains Eye Study (BMES) | Australia | White | 2003 | 3,654 | Arteriolar narrowing | Current blood pressure [19] |
Venular narrowing | Current blood pressure [19] | |||||
A/V ratio | Current blood pressure [19] | |||||
2004 | 2,335 | Arteriolar narrowing | Past and current systolic/diastolic blood pressure [20] | |||
AV ratio | Past diastolic and current systolic/diastolic blood pressure [20] | |||||
2004 | 1,319 | Arteriolar narrowing | Incident severe hypertension [21] | |||
A/V ratio | Incident severe hypertension [21] | |||||
2006 | 3,654 | Venular caliber increase | CHD death (men and women, 49–75 years) [22] | |||
Arteriolar caliber decrease | CHD death (women, 49–75 years) [22] | |||||
A/V ratio | CHD death (women, 49–75 years) [22] | |||||
Cardiovascular Health Study (CHS) | USA | White, black | 2002 | 2,405 | Arteriolar narrowing | Past and current blood pressure [23] |
A/V ratio | Current blood pressure [23] | |||||
2006 | 1,992 | Smaller arteriolar caliber | Incident CHD [24] | |||
Larger venular caliber | Incident CHD and stroke [24] | |||||
A/V ratio | Incident CHD [24] | |||||
Multi-Ethnic Study of Atherosclerosis (MESA) | USA | White, Hispanics, Black, Chinese | 2006 | 5,979 | Smaller arteriolar caliber | Current blood pressure [25] |
2009 | 2,583 | Narrower arteriolar diameter | Incident hypertension [26] | |||
Wider venular diameter | Incident hypertension [26] | |||||
2011 | 4,594 | Arteriolar narrowing | Incident CKD stage 3 (only whites) [27] | |||
Rotterdam Study | Netherlands | White | 2004 | 5,674 | Arteriolar diameter decrease | Current blood pressure [28] |
Venular diameter decrease | Current blood pressure [28] | |||||
A/V ratio | Current blood pressure [28] | |||||
2006 | 1,900 | Arteriolar narrowing | Incident hypertension [29] | |||
Venular narrowing | Incident hypertension [29] | |||||
A/V ratio | Incident hypertension [29] | |||||
2006 | 5,540 | Larger venular diameter | Incident stroke, cerebral infarction [30] | |||
2010 | 5,518 | Larger venular caliber | Incident stroke, cerebral infarction, intracerebral hemorrhage [31] | |||
Singapore Malay Eye Study (SiMES) | Singapore | Malay | 2008 | 3,019 | Smaller arteriolar caliber | Current blood pressure [32] |
2009 | 2,581 | Arteriolar narrowing | Prevalent CKD, micro-/macroalbuminuria [33] | |||
Singapore Prospective Study Program (SP2) | Singapore | Chinese, Malay, Indian | 2009 | 3,749 | Arteriolar caliber decrease | Current blood pressure [34] |
Venular caliber increase | Current blood pressure [34] | |||||
A/V ratio | Current blood pressure [34] | |||||
2009 | 3,602 | Arteriolar caliber decrease | Prevalent CKD [35] | |||
Sydney Childhood Eye Study | Australia | White, Chinese, and others | 2007 | 1,572 | Arteriolar narrowing | Current blood pressure [36] |
It has been repeatedly shown that retinal alterations are strongly correlated with past, current, and incident hypertension. In most of these large-scale studies, associations with directly assessed (generalized) arteriolar narrowing or a decreased A/V ratio, indirectly indicative of proposed arteriolar narrowing, and hypertension were reported (for details, see Table 28.2). In contrast, conflicting results according to retinal venules and hypertension were found. For example, in the Blue Mountains Eye Study, venular narrowing was associated with current hypertension [19]. In accordance, in the Rotterdam Study, venular narrowing was found to be predictive of current and incident blood pressure [28], but in the Multi-Ethnic Study of Atherosclerosis, venular widening was associated with incident hypertension [26]. While arteriolar narrowing can easily be harmonized with hypertension, it may be more difficult to explain why wider retinal venular caliber is associated with development of hypertension. A recent meta-analysis, comprising 10,229 patients without prevalent hypertension, diabetes, or CV disease, proposed that 2,599 patients developed new-onset hypertension during follow-up of 2.9–10 years. Both arteriolar narrowing (OR per 20 μm difference 1.29, 95 % CI 1.20–1.39) and venular widening (OR per 20 μm difference 1.14, 95 % CI 1.06–1.23) were independently associated with incident hypertension [37].
Importantly, in a population-based cohort comprising 1,572 children aged 6–8 years, each 10 mmHg increase of systolic BP was associated with arteriolar narrowing by 2.08 μm (95 % CI: 1.38–2.79, p < 0.0001), indicative that effects of elevated BP manifest early in life [36].
Regarding TOD data are even more limited. In 1,439 middle-aged African-Americans participants of the Atherosclerosis Risk in Communities Study A/V ratio was associated with measures of left ventricular hypertrophy, which was partly explained by additional CV risk factors and hypertension [13]. In contrast, in an Italian study comprising 386 untreated and treated hypertensive patients, no intergroup differences in A/V ratio was found between presence and absence of acknowledged TOD like left ventricular hypertrophy, carotid intima-media thickness, or microalbuminuria, hence indicating limited value of A/V ratio for identifying patients with high CV risk based on cardiac and extracardiac TOD [38].
There is also an ambiguous picture of arteriolar and venular diameter and different components of CV events. Regarding incident stroke, associations of both arteriolar narrowing and venular widening were reported in some studies, whereas in the Rotterdam Study, only an association of venular widening was found, but not for arteriolar narrowing [30]. Moreover, in the latter Rotterdam Study, venular widening was also associated with intracerebral hemorrhage [31]. These conflicting results are supported based on meta-analyses performed mainly by the META-EYE study group and published in the last years [18, 39].
These conflicting results of the individual components (with respect to arteriolar and venular diameter) have also to be taken into account, when interpreting reported findings about A/V ratio. An altered A/V ratio can be due to single and concurrent changes and their individual amount, and vice versa nonfindings can be seen, for example, by diverging changes.
28.3 Global Geometrical and Branching Parameters (Retinal Vascular Network)
The vasculature is a branching system, and alterations from optimal architecture are proposed to impair function and hence increased vascular damage. Thus, interest has gained on further developments in computer-assisted programs enabling the assessment of several quantitative parameters of retinal vascular network. Using these newly developed retinal vascular parameters, analysis of the Singapore Malay Eye Study has shown that a combination of smaller retinal vascular fractal dimensions (D f), proposed to be a global measure of the geometric complexity, and evidence of straighter retinal arterioles indicate poor BP control in treated hypertensive patients [40]. Therefore, retinal alterations can be assumed as pathophysiological markers not only for the severity of hypertension, but also on the effectiveness of drug therapy in hypertension.
Utilizing data from the multiethnic Singapore Prospective Study Program (SP2), the same group has shown that retinal D f was inversely correlated with BP level in all three ethnic groups. Notably, this was the case in patients with uncontrolled as well as untreated hypertension, but not in patients with controlled hypertension [41].
However, by applying again and again several new parameters, and analyzing these new parameters in the same studies, it is still missing the differentiation which is the most promising and reliable parameter to detect early retinal involvement in the clinical course of hypertension.
28.4 Scanning Laser Doppler Flowmetry (Dynamic)
Funduscopic photographs have the limitation that arteriolar and venular alterations cannot be quantified separately and the vascular wall precisely visualized. Moreover, the term remodeling if assessed in vivo takes two aspects into account, which were interrelated and indistinguishable, namely, morphological changes (i.e., rearrangement of vascular smooth muscle cells) as well as changes of the vascular tone (i.e., endothelial function). To overcome these limitations, one promising approach introduced by our study group about 10 years ago allows the dynamic assessment of both functional and structural parameters by using scanning laser Doppler flowmetry (SLDF) [42]. In brief, SLDF is performed in the juxtapapillary area of the right eye, 2–3 mm temporal superior of the optic nerve at 670 nm (Heidelberg Retina Flowmeter, Heidelberg Engineering, Germany). An arteriole (80–140 μm) of the superficial retinal layer in a retinal sample of 2.56 × 0.64 × 0.30 nm is scanned within 2 s (one systolic and one diastolic phase) and measured every 10 μm of this specific length of the arteriole. The confocal technique of the device ensures that only capillary flow of the superficial layer of 300 μm is measured. The outer arteriole diameter (AD) is measured by reflection images, and the lumen diameter (LD) is measured by perfusion images. Wall-to-lumen ratio (WLR) is calculated using the formula (AD – LD)/LD (Fig. 28.2). Analyses are performed offline with automatic full-field perfusion imaging analysis (AFFPIA) (SLDF Version 4.0 by Welzenbach with improved resolution) [43].
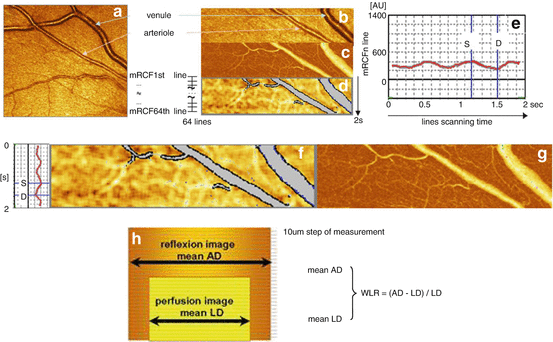

Fig. 28.2
Scanning laser Doppler flowmetry (SLDF). (a) Differentiation between retinal arteriole and venule (SLDF live image before measurement). (b) Scanned area – reflection image. (c) Scanned area – perfusion image. (d) Scanned area – corrected and analyzed flow image. (e) Pulse curve run as mean retinal capillary flow (RCF) and time plot.(f) Localization of systolic and diastolic RCF on the image d. (g) Localization of systolic and diastolic RCF on the image c. (h) Calculation of wall-to-lumen ratio (WLR)
It is noteworthy to mention that assessing both retinal function and structure by SLDF does not require applying any mydriatic drug, which is not only important from the scientific point of view – local application of tropicamide profoundly affects the retinal perfusion [44], but also for patient management perspective (i.e., no constriction of daily routine).
28.5 Retinal Capillary Flow
Due to its common origin from the internal carotid artery, the retinal microcirculation is morphologically and functionally related to the cerebral circulation [45].
Further dynamic information (e.g., basal nitric oxide [NO] activity) of the retinal capillaries can be assessed by measuring changes of retinal capillary flow (RCF) due to nonpharmacological and pharmacological tools. Flicker light increases at least in part via a NO-dependent mechanism and represents a nonpharmacological tool to investigate vasodilatory capacity of retinal capillaries. Notably, flicker light exposure has no effects on systemic BP, thereby minimizing potential systemic hemodynamic influences on RCF. Moreover, basal NO activity is assessed by administration of the NO synthase inhibitor NG-monomethyl-L-arginine (L-NMMA). Findings in normo- and hypertensive patients are summarized in Table 28.3.
Table 28.3
Studies of our research group, analyzing hypertensive patients and/or patients with cardiovascular event, using scanning laser Doppler flowmetry to assess early vascular changes (in chronological order)
Reference | Patients | Retinal endothelial function | Retinal arteriolar structure |
|---|---|---|---|
Delles et al. [42]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|