Resynchronization Therapy in Pediatric and Congenital Heart Disease Patients
Eric S. Silver
Anne M. Dubin
Cardiac resynchronization therapy is a powerful tool for the treatment of patients with cardiac failure and evidence of left ventricular dyssynchrony.1 Current guidelines advise biventricular pacing for patients with an ejection fraction of less than 35%, QRS width > 120 msec, and New York Heart Association (NYHA) Class III or IV heart failure despite maximal medical therapy.2 While this constitutes a significant percentage of the adult heart failure population, children with heart failure and patients with congenital heart disease rarely meet these criteria.3 The typical pediatric patient in whom resynchronization has been attempted are those with congenital heart disease (70%), followed by primary cardiomyopathy and congenital complete atrioventricular block.4 Studies to identify the pediatric population who benefit from resynchronization have been ongoing, albeit with significantly smaller numbers of patients than those typically encountered in the adult population.
In the pediatric literature, the terms cardiac resynchronization therapy (CRT) and multisite pacing (MSP) have gained favor over biventricular pacing due to variability in patient anatomy, and will be used interchangeably throughout this chapter.
FROM WHENCE TO PACE
It has long been known that, while the right ventricular (RV) apex provides an accessible target for lead placement in the majority of patients, the electrical activation sequence produced when pacing from this location can have deleterious effects.5 Chronic RV apical pacing causes RV dyssynchronous contraction and decreased left ventricular function. Both acute6 and chronic7,8,9 deleterious hemodynamic effects have been noted.
The search for an ideal pacing location to minimize ventricular remodeling and the likelihood of developing a pacing-related cardiomyopathy remains ongoing. Various alternative pacing sites have been put forth, including the His region,10,11,12 right ventricular outflow tract,13,14,15 right ventricular septum,16 the left ventricle,6 or some combination of these. Permanent His bundle pacing would likely be an ideal target; however, to date, technical issues including lead stability have thus far precluded this option as a routine site.
In pediatric patients, epicardial pacing continues to represent a significant percentage of the pacing population due to small patient size or presence of intracardiac shunts,4 allowing for a broader choice for pacing location in these patients. Left ventricular (LV) apical pacing has been offered as a potential alternative to right ventricular pacing.6,17,18 Vanagt et al. demonstrated synchronous LV endocardial activation in canines utilizing LV apical pacing. They went on to demonstrate improved acute hemodynamic parameters utilizing LV apical pacing as compared to RV apical and LV free wall pacing in patients following repair of congenital heart defects.6,18 These patients retained intact AV conduction; therefore the pacing protocol utilized relatively short AV intervals in order to minimize the patients’ intrinsic conduction.
Animal models of complete AV block have been utilized to test pacing from various sites as well as biventricular, or multisite, pacing. Cojoc et al. used an immature swine model to evaluate systolic mechanics and echocardiographic measures of dyssynchrony following AV nodal ablation.19 With AV sequential pacing they demonstrated improved systolic mechanics by measuring pressure volume loops with impedance catheters from within the LV cavity while pacing either the LV apex or biventricular pacing, as compared to controls with RV apical pacing. These studies suggest that prophylactic LV apical pacing may be beneficial in avoiding the long-term dyssynchrony seen in a subset of pediatric patients.
TARGET POPULATION FOR CRT
The appropriate patient for biventricular pacing has evolved since the concept was first introduced into the clinical arena. While current data supports criteria for the use of biventricular pacing in the adult heart failure population,2 the ideal pediatric patient is less clear. The following three major patient groups have shown improvement with resynchronization therapy: patients with congenital heart disease, those with congenital complete heart block, and those with cardiomyopathy.
Cardiac resynchronization offers a potential benefit to patients with failing palliation for various congenital heart lesions. Heart failure in this population offers a particularly
poor prognosis, as the relative risk for death following transplant in patients born with congenital heart disease ranges from 1.9 to 5.6 at one year as compared to pediatric patients with a primary cardiomyopathy, with the variability related to patient age and the need for ECMO at the time of heart transplantation.20 Approximately 45% of pediatric patients who received a cardiac transplant in North America between January 2000 and June 2006 had a diagnosis of congenital heart disease.20
poor prognosis, as the relative risk for death following transplant in patients born with congenital heart disease ranges from 1.9 to 5.6 at one year as compared to pediatric patients with a primary cardiomyopathy, with the variability related to patient age and the need for ECMO at the time of heart transplantation.20 Approximately 45% of pediatric patients who received a cardiac transplant in North America between January 2000 and June 2006 had a diagnosis of congenital heart disease.20
Resynchronization has been tried in the following specific lesions: patients with failing right ventricles, patients with systemic right ventricles and biventricular anatomy, and those with single ventricle physiology.
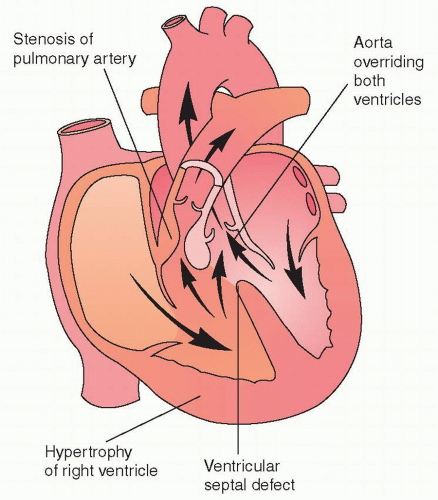
As opposed to left-sided heart failure, right-sided heart failure is one of the major sequelae of congenital heart disease palliation. Tetralogy of Fallot is the most commonly used example of this problem. Tetralogy of Fallot (TOF) is caused by anterior deviation of the conal septum, leading to pulmonary stenosis, aortic override, and a ventricular septal defect (Fig. 10.1). Surgical repair of Tetralogy of Fallot is almost universally associated with the development of a right bundle branch block pattern on the electrocardiogram,21 related to the resection of infundibular tissue22 and long-term issues with pulmonary insufficiency and right ventricular dilatation. This combination of right ventricular pressure load at an early age and right ventricular volume load after repair can lead to right ventricular failure and arrhythmias. There is significant echocardiographic evidence of right ventricular dyssynchrony in this population.23 This suggests that right ventricular resynchronization (as opposed to left ventricular) may be a reasonable target for this population.
Patients with systemic right ventricles are also known to be at risk of developing systemic (right ventricular) heart failure. Patients with a systemic right ventricle in the setting of a biventricular heart include both those with D-Transposition of the great arteries (D-TGA) in whom repair was prior to the era of the Arterial Switch procedure and those with L-Transposition of the great arteries (L-TGA).
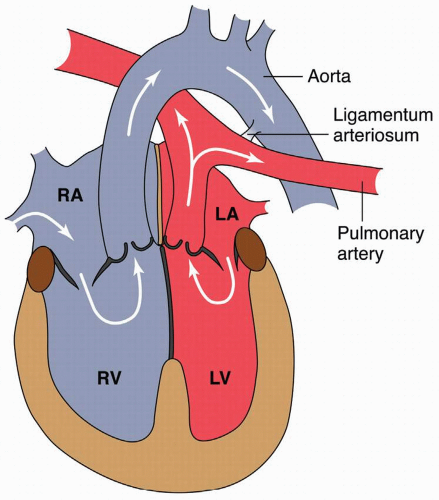
D-TGA is a disorder of conoventricular septation with the right ventricle pumping to the aorta and left ventricle to the pulmonary artery (Fig. 10.2). Prior to the era of the Arterial Switch procedure, which became the standard of care in the 1980s, the Senning and Mustard procedures were routinely performed. Both involved constructing an intra-atrial baffle to deliver deoxygenated venous blood to the left ventricle and oxygenated blood to the systemic right ventricle (Fig. 10.3). These repairs have long-term morbidities including sinus node dysfunction, atrial arrhythmias, baffle obstructions, and systemic right ventricular failure.24,25,26 Heart failure has been noted in 22% of patients following the Mustard procedure for D-TGA.26
Twenty-nine patients (21 ± 3.5 years of age) with a history of D-TGA who had undergone Mustard (n=21) or Senning (n=7) procedures were evaluated for evidence of mechanical dyssynchrony utilizing tissue Doppler echocardiography.27 Despite the fact that all patients were NYHA functional class I (n = 25) or II (n = 4), 32% still exhibited evidence of mechanical dyssynchrony (defined as a right inraventricular mechanical delay of >49 msec).
L-TGA is an anomaly frequently associated with other cardiovascular defects, such as ventricular septal defects,28 and the repair will often depend on concomitant cardiovascular
anomalies (Fig. 10.4). These patients have a risk of systemic right ventricular failure over time.26 Sixty-seven percent of patients with associated cardiac lesions and 25% of those without will go on to develop right ventricular failure by age 45.29 As patients with L-TGA have a 2% per year incidence of spontaneous complete AV block,30 two issues arise: pacing patients with systemic right ventricular dyssynchrony and normal intrinsic AV conduction, and pacing those with complete heart block.
anomalies (Fig. 10.4). These patients have a risk of systemic right ventricular failure over time.26 Sixty-seven percent of patients with associated cardiac lesions and 25% of those without will go on to develop right ventricular failure by age 45.29 As patients with L-TGA have a 2% per year incidence of spontaneous complete AV block,30 two issues arise: pacing patients with systemic right ventricular dyssynchrony and normal intrinsic AV conduction, and pacing those with complete heart block.
 Get Clinical Tree app for offline access 
|