Restrictive disorders
Restrictive disorders affect the interstitium of the lungs (alveoli, blood vessels, and surrounding tissues). In restrictive disorders, a decrease in the lung capacity results from the inability of the lungs to expand and relax at the rate and completeness the diaphragm and intercostal muscles demand. With restrictive pulmonary disease, thickening of the lung tissues can occur and will result in a decrease in arterial oxygen levels.
Restrictive disorders reduce vital capacity and the functional residual capacity, but airflow remains normal. Conditions such as pulmonary fibrosis damage lung tissue and result in loss of elasticity. Typically, the onset of these disorders is slow and insidious, and dyspnea is the most common symptom.
Restrictive disorders include acute respiratory distress syndrome, acute respiratory failure, asbestosis, asthma, atelectasis, berylliosis, coal workers’ pneumoconiosis, idiopathic pulmonary fibrosis, respiratory distress syndrome, sarcoidosis, and silicosis.
ACUTE RESPIRATORY DISTRESS SYNDROME
Acute respiratory distress syndrome (ARDS) is a form of pulmonary edema that can quickly lead to acute respiratory failure. It’s also known as shock, stiff, white, wet, or Da Nang lung. It may follow direct or indirect lung injury.
Trauma is the most common cause of ARDS. Trauma-related factors, such as fat emboli, sepsis, shock, pulmonary contusions,
traumatic tissue injury, and multiple transfusions increase the likelihood of microemboli developing.
traumatic tissue injury, and multiple transfusions increase the likelihood of microemboli developing.
AGE AWARE
Acute respiratory distress syndrome (ARDS) is a significant cause of mortality in children. Children at risk for ARDS include those with infections that cause fluid accumulation in the lungs. Children with any type of lung infection should receive prompt treatment to avoid this serious condition.
Other common causes of ARDS include anaphylaxis, aspiration of gastric contents, diffuse pneumonia (especially viral), drug overdose (for example, heroin, aspirin, and ethchlorvynol), idiosyncratic drug reaction (to ampicillin and hydrochlorothiazide [HydroDIURIL]), inhalation of noxious gases (such as nitrous oxide, ammonia, and chlorine), near-drowning, and ventilator-induced oxygen toxicity.
Less common causes of ARDS include coronary artery bypass grafting, hemodialysis, leukemia, acute miliary tuberculosis, pancreatitis, thrombotic thrombocytopenic purpura, uremia, and venous air embolism. (See ARDS in children.)
Pathophysiology
Increased permeability of the alveolocapillary membranes allows fluid to accumulate in the lung interstitium, alveolar spaces, and small airways, causing the lung to stiffen. This impairs ventilation, reducing oxygenation of pulmonary capillary blood. The disorder is difficult to recognize and can prove fatal within 48 hours of onset if not promptly diagnosed and treated. (See What happens in ARDS.)
This four-stage syndrome can progress to intractable and fatal hypoxemia; however, patients who recover may have little or no permanent lung damage.
In some patients, the syndrome may coexist with disseminated intravascular coagulation (DIC). It remains unclear whether ARDS stems from DIC or develops independently. Patients with three concurrent ARDS risk factors have an 85% probability of developing ARDS.
UP CLOSE
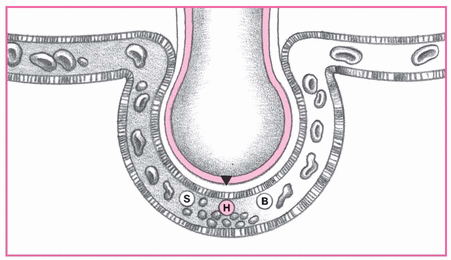
These illustrations depict the process and progress of acute respiratory distress syndrome (ARDS).
1. The body responds to insult.
Injury reduces normal blood flow to the lungs, allowing platelets to aggregate. These platelets release such substances as serotonin (S), bradykinin (B) and, especially, histamine (H) that inflame and damage the alveolar membrane and later increase capillary permeability. At this early stage, signs and symptoms of ARDS are undetectable.
|
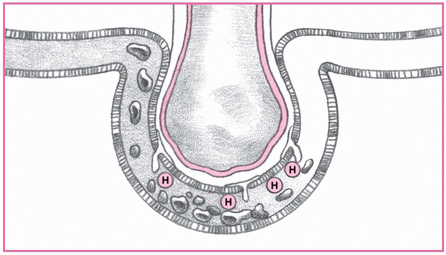
2. Fluid shift causes symptoms.
Increased capillary permeability allows fluid to shift into the interstitial space. As a result, the patient may experience tachypnea, dyspnea, and tachycardia.
|
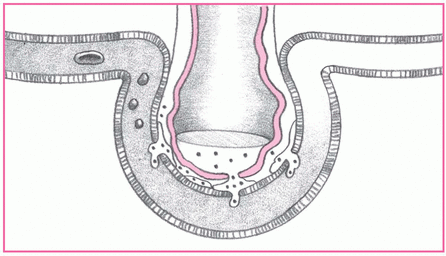
3. Pulmonary edema results.
As capillary permeability continues to increase, shifting of proteins and fluid increases interstitial osmotic pressure and causes pulmonary edema. At this stage, the patient may experience increased tachypnea, dyspnea, and cyanosis. Hypoxia (usually unresponsive to increased fraction of inspired oxygen), decreased pulmonary compliance, and crackles and rhonchi may also develop.
|
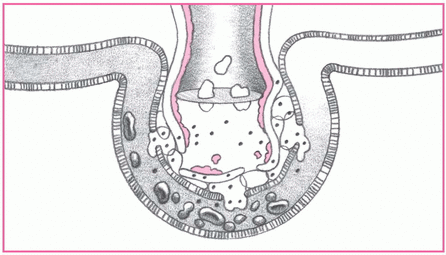
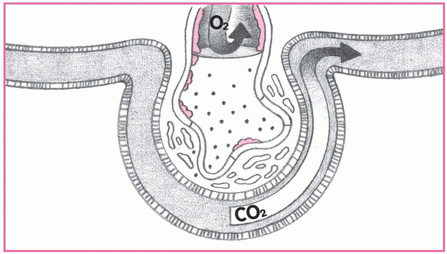
4. Alveoli collapse.
Fluid in the alveoli and decreased blood flow damage surfactant in the alveoli, reducing the cells’ ability to produce more surfactant. Without surfactant, alveoli collapse, impairing gas exchange. Look for thick, frothy sputum and marked hypoxemia with increased respiratory distress.
|
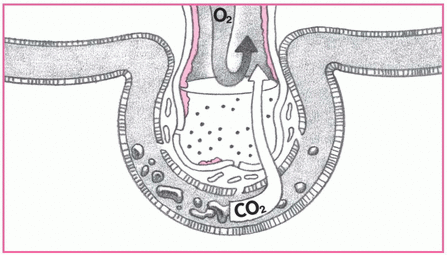
5. Gas exchange slows.
The patient breathes faster, but sufficient oxygen (O2) can’t cross the alveolocapillary membrane. Carbon dioxide (CO2), however, crosses more easily and is lost with every exhalation. Both O2 and CO2 levels in the blood decrease. Look for increased tachypnea, hypoxemia, and hypocapnia.
|
6. Metabolic acidosis occurs.
Pulmonary edema worsens. Meanwhile, inflammation leads to fibrosis, which further impedes gas exchange. The resulting hypoxemia leads to metabolic acidosis. At this stage, look for increased partial pressure of arterial carbon dioxide; decreased bicarbonate level, pH, and partial pressure of arterial oxygen; and mental confusion.
|
Complications
Metabolic and respiratory acidosis
Respiratory and cardiac arrest
Assessment findings
As you conduct your assessment, be alert for the patient’s particular stage of ARDS. Each has its typical signs and symptoms.
STAGE I
The patient may complain of dyspnea, especially on exertion.
Respiratory and pulse rates are normal to high.
Auscultation may reveal diminished breath sounds.
STAGE II
Respiratory distress becomes more apparent.
The patient may use accessory muscles to breathe and may appear pallid, anxious, and restless.
He may have a dry cough with thick, frothy sputum and bloody, sticky secretions.
Palpation may disclose cool, clammy skin.
Tachycardia and tachypnea may accompany elevated blood pressure.
He may have a change or decrease in mental status.
Auscultation may reveal basilar crackles. (Stage II signs and symptoms may be incorrectly attributed to other causes such as multiple traumas.)
The patient needs intubation and ventilation.
STAGE III
The patient struggles to breathe.
Vital signs reveal tachypnea (more than 30 breaths/minute), tachycardia with arrhythmias (usually premature ventricular contractions), and a labile blood pressure. Inspection may reveal a productive cough and pale, cyanotic skin.
He may demonstrate a change or decrease in mental status. Auscultation may disclose crackles and rhonchi.
STAGE IV
Acute respiratory failure with severe hypoxia occurs.
The patient’s mental status deteriorates, and he may become comatose.
His skin appears pale and cyanotic.
Spontaneous respirations aren’t evident. Bradycardia with arrhythmias accompanies hypotension.
Metabolic acidosis and respiratory acidosis develop.
When ARDS reaches this stage, the patient is at high risk for fibrosis.
Pulmonary damage becomes life-threatening.
Diagnostic test results
Arterial blood gas (ABG) analysis (with the patient breathing room air) initially shows a reduced partial pressure of arterial oxygen (PaO2) of less than 60 mm Hg and a decreased partial pressure of arterial carbon dioxide (PaCO2) of less than 35 mm Hg. Hypoxemia despite increased supplemental oxygen is the hallmark of ARDS. The resulting blood pH usually
reflects respiratory alkalosis. As ARDS worsens, ABG values show respiratory acidosis (increasing PaCO2 [greater than 45 mm Hg]) and metabolic acidosis (decreasing bicarbonate levels [less than 22 mEq/L]) and declining PaO2 despite oxygen therapy. The PaO2 to fraction of inspired oxygen ratio is 200 mm Hg or less, regardless of the positive end-expiratory pressure (PEEP) level.
Differential diagnosis must rule out cardiogenic pulmonary edema, pulmonary vasculitis, and diffuse pulmonary hemorrhage. Etiologic tests may involve sputum analyses (including Gram stain and culture and sensitivity), blood cultures (to identify infectious organisms), toxicology tests (to screen for drug ingestion), and various serum amylase tests (to rule out pancreatitis).
Pulmonary artery catheterization helps to identify the cause of pulmonary edema by measuring pulmonary artery wedge pressure (PAWP). This procedure also allows the collection of samples of pulmonary artery and mixed venous blood that shows decreased oxygen saturation, reflecting tissue hypoxia. Normal PAWP values in ARDS are 12 mm Hg or less.
Serial chest X-rays in early stages show bilateral infiltrates. In later stages, findings demonstrate lung fields with a ground-glass appearance and, eventually (with irreversible hypoxemia), “whiteouts” of both lung fields.
Treatment
Therapy focuses on correcting the cause of ARDS, if possible, and preventing the progression of life-threatening hypoxemia and respiratory acidosis. Supportive care consists of giving humidified oxygen through a tightly fitting mask, which facilitates the use of continuous positive airway pressure (CPAP). However, this therapy alone seldom fulfills the patient’s ventilatory requirements. If the patient’s hypoxemia doesn’t subside with this treatment, he may require intubation, mechanical ventilation, and PEEP. Prevention of health care-related infections is also important. Other supportive measures include fluid restriction, diuretic therapy, and correction of electrolyte and acid-base imbalances.
When a patient with ARDS needs mechanical ventilation, sedatives, opioids, or neuromuscular blockers (such as vecuronium [Norcuron]) may be ordered to minimize restlessness (and thereby oxygen consumption and carbon dioxide production) and facilitate ventilation.
When ARDS results from fatty emboli or a chemical injury, a short course of high-dose corticosteroids may help if given early. Treatment with sodium bicarbonate may be necessary to reverse severe metabolic acidosis, and fluids and vasopressors may be needed to maintain blood pressure. Nonviral infections require treatment with antimicrobial drugs.
Nursing interventions
Frequently assess the patient’s respiratory status. Be alert for inspiratory retractions. Note respiratory rate, rhythm, and depth. Watch for dyspnea and accessory muscle use. Listen for adventitious or diminished breath sounds. Check for clear, frothy sputum (indicating pulmonary edema).
Evaluate and document the patient’s level of consciousness, noting confusion or mental sluggishness.
Be alert for signs of treatment-induced complications, including arrhythmias, DIC, GI bleeding, infection, malnutrition, paralytic ileus, pneumothorax, pulmonary fibrosis, renal failure, thrombocytopenia, and tracheal stenosis.
Maintain a patent airway by suctioning. Use sterile, nontraumatic technique. Ensure adequate humidification to help liquefy tenacious secretions.
Closely monitor heart rate and blood pressure. Watch for arrhythmias that may result from hypoxemia, acid-base disturbances, or electrolyte imbalance.
With pulmonary artery catheterization, know the desired PAWP level; check readings often, and watch for decreasing mixed venous oxygen saturation. Change dressings according to facility guidelines, using strict aseptic technique.
Monitor serum electrolyte levels, and correct imbalances. Measure intake and output. Weigh the patient daily.
DISCHARGE TEACHING
Explain acute respiratory distress syndrome (ARDS) to the patient and his family. Tell them what signs and symptoms may occur, and review the treatment that may be required.
Review all prescribed drugs, including their names, dosages, actions, adverse affects, and special insructions.
Teach about the use of home oxygen.
Tell the recuperating patient that recovery takes some time and that he’ll feel weak for a while. Emphasize the importance of a pulmonary rehabilitation program.
Be ready to provide CPAP to the patient with severe hypoxemia.
Give sedatives, as ordered, to reduce restlessness. Monitor and record the patient’s response to medication.
Because PEEP may lower cardiac output, check for hypotension, tachycardia, and decreased urine output. To maintain PEEP, suction only as needed. High-frequency jet ventilation and pressure-controlled ventilation may also be required.
Reposition the patient often. Proning—maintaining the patient in a prone position for 6 or more hours per day—may be used to improve ventilation-perfusion mismatch.
Record an increase in secretions, temperature, or hypotension that may indicate a deteriorating condition.
Monitor the patient’s nutritional intake, and record caloric intake. Give tube feedings and parenteral nutrition as ordered. Plan patient care to allow periods of uninterrupted sleep. To promote health and prevent fatigue, arrange for alternate periods of rest and activity.
Maintain joint mobility by performing passive range-ofmotion (ROM) exercises. If possible, help the patient perform active ROM exercises.
Provide meticulous skin care. To prevent skin breakdown, reposition the endotracheal tube from side to side every 24 hours.
Provide emotional support. Answer the patient’s and family’s questions as completely as possible to allay their fears and concerns.
Watch for and immediately report all respiratory changes in the patient with injuries that may adversely affect the lungs— especially in the first few days after the injury when the patient’s condition may appear to be improving.
Provide alternative communication means for the patient on mechanical ventilation. (See ARDS teaching topics.)
ACUTE RESPIRATORY FAILURE
When the lungs can’t adequately maintain arterial oxygenation or eliminate carbon dioxide, acute respiratory failure occurs. If not checked and treated, the condition leads to tissue hypoxia. In patients with essentially normal lung tissue, acute respiratory failure usually produces a partial pressure of arterial carbon dioxide (PaCO2) greater than 50 mm Hg and a partial pressure of arterial oxygen (PaO2) less than 50 mm Hg.
These limits, however, don’t apply to patients with chronic obstructive pulmonary disease (COPD). These patients consistently have high PaCO2 (hypercapnia) and low PaO2 (hypoxemia) levels. For patients with COPD, only acute deterioration in arterial blood gas (ABG) values and corresponding clinical deterioration signal acute respiratory failure.
Pathophysiology
Respiratory failure results from impaired gas exchange. Conditions associated with alveolar hypoventilation, ventilation-perfusion mismatch, and intrapulmonary (right-to-left) shunting can cause acute respiratory failure if left untreated. Acute respiratory failure may develop in patients with COPD from any condition that increases the work of breathing and decreases the respiratory drive. These conditions can result from respiratory tract infection (such as bronchitis or pneumonia), bronchospasm, or accumulated secretions secondary to cough suppression. Other common causes are related to ventilatory failure, in which the brain fails to direct respiration, and gas exchange failure,
in which respiratory structures fail to function properly. (See What happens in acute respiratory failure.)
in which respiratory structures fail to function properly. (See What happens in acute respiratory failure.)
UP CLOSE
Three major malfunctions account for impaired gas exchange and subsequent acute respiratory failure: alveolar hypoventilation, ventilation-perfusion ([V with dot above]/[Q with dot above]) mismatch, and intrapulmonary (right-to-left) shunting.
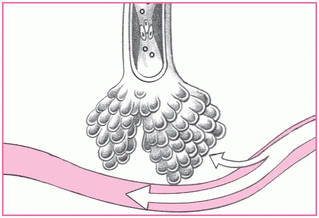
Alveolar hypoventilation
Decreased oxygen saturation may result when chronic airway obstruction reduces alveolar minute ventilation. In such cases, partial pressure of arterial oxygen (PaO2) levels fall and partial pressure of arterial carbon dioxide levels rise, and hypoxia results.
|
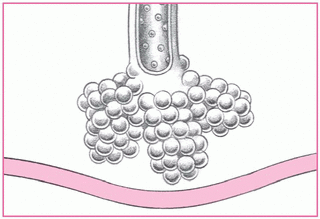
[V with dot above]/[Q with dot above] mismatch
The most common cause of hypoxemia-imbalances in ventilation and perfusion-occur when conditions such as pulmonary embolism or acute respiratory distress syndrome interrupt normal gas exchange in a specific lung region. Either too little ventilation with normal blood flow or too little blood flow with normal ventilation may cause the imbalance. Whichever happens, the result is the same: PaO2 levels fall.
|
Right-to-left shunting
Untreated ventilation or perfusion imbalances can lead to right-to-left shunting, in which blood passes from the heart’s right side to its left without being oxygenated.
|
Implications
The hypoxemia and hypercapnia characteristic of respiratory failure stimulate strong compensatory responses by all body systems, including the respiratory, cardiovascular, and central nervous systems.
In response to hypoxemia, for example, the sympathetic nervous system triggers vasoconstriction, increases peripheral resistance, and boosts the heart rate.
The body responds to hypercapnia with cerebral depression, hypotension, circulatory failure, and an increased heartbeat and cardiac output. Hypoxemia or hypercapnia (or both) cause the brain’s respiratory control center to first increase respiratory depth (tidal volume) and then increase the respiratory rate. As respiratory failure worsens, intercostal, supraclavicular, and suprasternal retractions may also occur.
CAUSES OF RESPIRATORY FAILURE
Problems with the brain, lungs, muscles and nerves, or pulmonary circulation can impair gas exchange and cause respiratory failure. Here’s a list of conditions that can cause respiratory failure.
Brain
Anesthesia
Cerebral hemorrhage
Cerebral tumor
Drug overdose
Head trauma
Skull fracture
Lungs
Acute respiratory distress syndrome
Asthma
Chronic obstructive pulmonary disease
Cystic fibrosis
Flail chest
Massive bilateral pneumonia
Sleep apnea
Tracheal obstruction
Muscles and nerves
Amyotrophic lateral sclerosis
Guillain-Barré syndrome
Multiple sclerosis
Muscular dystrophy
Myasthenia gravis
Polio
Spinal cord trauma
Pulmonary circulation
Heart failure
Pulmonary edema
Pulmonary embolism
Other causes of acute respiratory failure include:
airway irritants, such as smoke or fumes
cardiovascular disorders (myocardial infarction, heart failure, or pulmonary emboli)
central nervous system depression owing to head trauma or injudicious use of sedatives, opioids, tranquilizers, or oxygen
endocrine or metabolic disorders, such as myxedema or metabolic acidosis
thoracic abnormalities, such as chest trauma, pneumothorax, or thoracic or abdominal surgery
noncompliance with prescribed bronchodilator or corticosteroid therapy. (See Causes of respiratory failure.)
Complications
Tissue hypoxia
Metabolic acidosis
Respiratory and cardiac arrest
Assessment findings
Because acute respiratory failure in COPD is life-threatening, you probably don’t have time to conduct an in-depth patient interview. Instead, rely on family members or the patient’s medical records to discover the precipitating incident.
On inspection, note cyanosis of the oral mucosa, lips, and nail beds; nasal flaring; and ashen skin.
You may observe the patient yawning and using accessory muscles to breathe.
He may appear restless, anxious, depressed, lethargic, agitated, confused, or combative.
The patient usually exhibits tachypnea, which signals impending respiratory failure.
Palpation may reveal cold, clammy skin and asymmetrical chest movement, which suggests pneumothorax.
If tactile fremitus is present, notice that it decreases over an obstructed bronchi or pleural effusion and increases over consolidated lung tissue.
Percussion—especially in patients with COPD—reveals hyperresonance.
If acute respiratory failure results from atelectasis or pneumonia, percussion usually produces a dull or flat sound.
Auscultation typically reveals diminished breath sounds.
In patients with pneumothorax, breath sounds may be absent. In other cases of respiratory failure, you may hear such adventitious breath sounds as wheezes (in asthma) and rhonchi (in bronchitis).
If you hear crackles, suspect pulmonary edema as the cause of respiratory failure. (See Identifying respiratory failure.)
Diagnostic test results
ABG analysis is the key to the diagnosis (and subsequent treatment) of acute respiratory failure. Progressively deteriorating ABG values and pH compared with the patient’s normal
values strongly suggest acute respiratory failure. In the patient with essentially normal lung tissue, a pH of less than 7.35 usually indicates acute respiratory failure. In the patient with COPD, the pH deviation from the normal value is even lower.
IDENTIFYING RESPIRATORY FAILURE
Use the following measurements to identify respiratory failure:
Vital capacity less than 15 ml/kg
Tidal volume less than 3 ml/kg
Negative inspiratory force less than -25 cm H2O
Respiratory rate more than twice the normal rate
Diminished partial pressure of arterial oxygen despite increased fraction of inspired oxygen
Elevated partial pressure of arterial carbon dioxide with pH less than 7.25
Blood tests such as white blood cell count are used to detect underlying causes. Abnormally low hematocrit and decreased hemoglobin level signal blood loss, which indicates decreased oxygen-carrying capacity.
Chest X-rays are used to identify underlying pulmonary diseases or conditions, such as emphysema, atelectasis, lesions, pneumothorax, infiltrates, and effusions.
Electrocardiography can demonstrate arrhythmias. Common electrocardiographic patterns point to cor pulmonale and myocardial hypoxia.
Pulmonary artery catheterization helps to distinguish pulmonary and cardiovascular causes of acute respiratory failure and is used to monitor hemodynamic pressures.
Pulse oximetry reveals decreasing arterial oxygen saturation but isn’t as reliable as ABG analysis.
Serum electrolyte findings vary. Hypokalemia may result from compensatory hyperventilation, the body’s attempt to correct alkalosis; hypochloremia usually occurs in metabolic alkalosis.
Additional tests, such as blood culture, Gram stain, and sputum culture, may be used to identify the pathogen.
Treatment
Acute respiratory failure in the patient with COPD is an emergency. The patient needs cautious oxygen therapy (nasal prongs or a Venturi mask) to increase his PaO2. If significant respiratory acidosis persists, mechanical ventilation with an endotracheal (ET) or tracheostomy tube may be needed. High-frequency ventilation may be initiated if the patient doesn’t respond to conventional mechanical ventilation. Treatment routinely includes antibiotics (for infection), bronchodilators and, possibly, corticosteroids.
If the patient also has cor pulmonale and decreased cardiac output, fluid restrictions and administration of positive inotropic agents, vasopressors, and diuretics may be ordered.
Nursing interventions
Orient the patient and his family to the treatment unit. Most patients with acute respiratory failure receive intensive care. Helping the patient understand procedures, sounds, and sights may ease his anxiety.
To reverse hypoxemia, give oxygen at appropriate concentrations to maintain PaO2 at a minimum pressure range of 50 to 60 mm Hg. The patient with COPD usually needs only small amounts of supplemental oxygen. Watch for a positive response, such as improved breathing, color, and ABG values.
Maintain a patent airway. If your patient retains carbon dioxide, encourage him to cough and breathe deeply with pursed lips. If he’s alert, have him use an incentive spirometer.
If he’s intubated and lethargic, reposition him every 1 to 2 hours. Use postural drainage and chest physiotherapy to help clear secretions.
Observe the patient closely for respiratory arrest. Auscultate chest sounds. Monitor ABG values and vital signs, and report changes immediately. Notify the physician of deterioration in oxygen saturation levels detected by pulse oximetry.
Watch for treatment complications, especially oxygen toxicity and acute respiratory distress syndrome.
Frequently monitor vital signs. Note and report an increasing pulse rate, increasing or decreasing respiratory rate, declining blood pressure, or febrile state.
Monitor and record serum electrolyte levels carefully. Take steps to correct imbalances. Monitor fluid balance by recording the patient’s intake and output and daily weight.
Check the cardiac monitor for arrhythmias.
Perform oral hygiene measures frequently.
Apply soft wrist restraints for the confused patient, if needed. This prevents him from disconnecting the oxygen setup. However, remember that these restraints can increase anxiety, fear, and agitation.
Position the patient for comfort and optimal gas exchange. Place the call button within his reach.
Keep the patient in a normothermic state to reduce the body’s demand for oxygen.
Pace and group patient care activities to maximize the patient’s energy level and provide needed rest.
If the patient requires mechanical ventilation:
Check ventilator settings, cuff pressures, and ABG values often to ensure correct fraction of inspired oxygen (FIO2) settings, which are determined by ABG levels. Draw blood samples for ABG analysis 20 to 30 minutes after every change in the FIO2 setting or as ordered.
Suction the trachea as needed. Observe for a change in sputum quality, consistency, and color. Provide humidification to liquefy secretions.
Watch for complications of mechanical ventilation, such as reduced cardiac output, pneumothorax or other barotrauma, increased pulmonary vascular resistance, diminished urine output, increased intracranial pressure, and GI bleeding.
Routinely assess ET tube position and patency. Make sure the tube is placed properly and taped securely. Immediately after intubation, check for accidental intubation of the esophagus or the mainstem bronchus, which may have occurred during ET tube insertion. Also be alert for transtracheal or laryngeal perforation, aspiration, broken teeth, nosebleeds, arrhythmias, hypertension, and vagal reflexes such as bradycardia.
After tube placement, watch for complications, such as tube displacement, herniation of the tube’s cuff, respiratory infection, and tracheal malacia and stenosis.
DISCHARGE TEACHING
If applicable, teach the patient about the effects of smoking. Provide resources to help him stop smoking.
Review the patient’s medication regimen with him.
If the patient receives home oxygen therapy, review the use of the equipment.
Refer the patient for pulmonary rehabilitation, as ordered.
Teach the patient and his family the signs and symptoms of respiratory infection and to call the physician when they occur.
Identify reportable signs of respiratory infection.
Prevent infection by using sterile technique while suctioning and by changing ventilator tubing every 24 hours.
Prevent tracheal erosion that can result from an overinflated artificial airway cuff compressing the tracheal wall’s vasculature. Use the minimal-leak technique and a cuffed tube with high residual volume (low-pressure cuff), a foam cuff, or a pressure-regulating valve on the cuff. Measure cuff pressure every 8 hours.
Implement measures to prevent tissue necrosis. Position and maintain the nasotracheal tube midline within the nostrils and provide meticulous care. Periodically loosen the tape holding the tube to prevent skin breakdown. Reposition the ET tube from side to side, and retape as needed. Make sure that the ventilator tubing has adequate support.
Monitor for signs of stress ulcers, which are common in the intubated patient, especially in the intensive care unit. Inspect gastric secretions for blood, especially if the patient has a nasogastric tube or reports epigastric tenderness, nausea, or vomiting. Also monitor the patient’s hemoglobin level and hematocrit, and check all stools for blood. Give antacids or histamine receptor antagonists, as ordered.
Help the patient communicate without words. Offer him a pen and writing tablet, a word chart, or an alphabet board. (See Acute respiratory failure teaching topics.)
ASBESTOSIS
Asbestosis is characterized by diffuse interstitial pulmonary fibrosis resulting from prolonged exposure to airborne asbestos particles. Asbestosis may develop about 15 to 20 years after regular exposure to asbestos ceases. Asbestos exposure also causes pleural plaques and mesotheliomas of the pleura and the peritoneum. A potent cocarcinogen, asbestos heightens a cigarette smoker’s risk of lung cancer. In fact, an asbestos worker who smokes is 90 times more likely to develop lung cancer than a smoker who never worked with asbestos.
Asbestosis is a form of pneumonoconiosis. It follows prolonged inhalation of respirable asbestos fibers (about 50 microns long and 0.5 micron wide). Sources of exposure include asbestos mining and milling, the construction industry (where asbestos is used in a prefabricated form), and the fireproofing and textile industries. Asbestos is also used in the production of paints, plastics, and brake and clutch linings. Asbestos-related diseases develop in families of asbestos workers as a result of exposure to fibrous dust shaken off workers’ clothing at home. Such diseases develop in the general public as a result of exposure to fibrous dust or waste piles from nearby asbestos plants.
Pathophysiology
Asbestosis occurs when lung spaces become filled with asbestos fibers. The inhaled asbestos fibers travel down the airway and penetrate respiratory bronchioles and alveolar walls. Coughing attempts to expel the foreign matter. Mucus production and goblet cells are stimulated to protect the airway from the debris and aid in expectoration. Fibers then become encased in a brown, iron-rich proteinlike sheath in sputum or lung tissue, called asbestosis bodies. Chronic irritation by the fibers continues to affect the lower bronchioles and alveoli. The foreign material and inflammation swell airways, and fibrosis develops in response to chronic irritation. Interstitial fibrosis may develop in lower lung zones, affecting lung parenchyma and the pleurae. Raised hyaline plaques may form in the parietal pleura, the diaphragm,
and the pleura adjacent to the pericardium. Hypoxia develops as more alveoli and lower airways are affected.
and the pleura adjacent to the pericardium. Hypoxia develops as more alveoli and lower airways are affected.
Complications
Pulmonary fibrosis
Respiratory failure
Pulmonary hypertension
Cor pulmonale
Assessment findings
The patient typically relates a history of occupational, family, or neighborhood exposure to asbestos fibers.
The average exposure time is about 10 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree