Restrictive Cardiomyopathy, Endocardial Fibrosis, and Eosinophilic Endomyocarditis
Fabio R. Tavora, M.D., Ph.D.
Allen P. Burke, M.D.
Restrictive Heart Disease
Restrictive heart disease refers to hemodynamic cardiac alterations that result from increased stiffness of the ventricles (diastolic dysfunction) causing impaired ventricular filling with preserved systolic function. The decrease in ventricular compliance is secondary to infiltrative disease of the myocardium (most frequently amyloid), endocardial disease, or nonischemic cardiomyopathy.
“Idiopathic” restrictive cardiomyopathy generally denotes today a form of nonischemic cardiomyopathy that may overlap genetically and clinically with dilated cardiomyopathy (see Chapter 19) but is marked primarily by restrictive hemodynamics. Primary acquired endocardial disease in adults is generally classified as tropical (idiopathic endocardial fibrosis) or eosinophilic. Congenital endocardial fibrosis is considered a form of infantile dilated cardiomyopathy (see Chapter 23).
Hemodynamic Findings
Because of increased ventricular stiffness, there is reduced diastolic ventricular volume with normal or near-normal systolic function (ejection fraction) and wall thickness. Ventricular pressures rise markedly with small increases in volume, and left ventricular end-diastolic volume is <100 mL/m2 with pressures >18 mm Hg. The atria are dilated with nonhypertrophied, nondilated ventricles. Pressure tracings show a characteristic diastolic dip and a plateau or a square root sign. Atrial fibrillation is noted in three-fourths of patients.1
Idiopathic Restrictive Cardiomyopathy
Genetic Findings
Patients with familial restrictive cardiomyopathy have familial phenotypes and genetic alterations that reflect hypertrophic, dilated, and noncompaction-type cardiomyopathy. One-third have sarcomere protein gene mutations involving cardiac troponin I gene (TNNI3), troponin T (TNNT2) desmin (DES), and alpha-cardiac actin (ACTC).2,3 Recently, mutations in TNNI3 (cardiac troponin I type 3) have been described in children with restrictive cardiomyopathy.4,5
Restrictive cardiomyopathy may be a manifestation of the myofibrillar myopathies, which are characterized by disintegration of the Z-disk, accumulation of cytoplasmic myofibrillar osmiophilic bodies ultrastructurally, and sometimes congophilic material on skeletal muscle biopsies. Myofibrillar myopathies may also manifest as arrhythmogenic and dilated cardiomyopathy.6 Mutations involved in myofibrillar myopathies include desmin, αB-crystallin, myotilin, ZASP, filamin C, and BAG3. Of these, desmin, αB-crystallin, and BAG3 have been implicated in cardiomyopathy.7
Gross Pathologic Findings
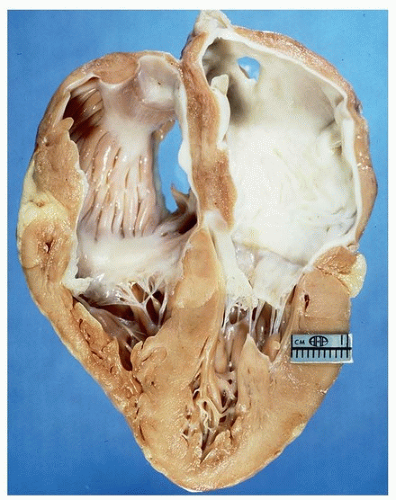
Grossly, the ventricles are relatively normal, but there is marked biatrial dilatation (Fig. 162.1).
Histologic Findings
The histologic features of idiopathic restrictive cardiomyopathy are nonspecific, including interstitial fibrosis and myocyte hypertrophy. Most cases with histologic evaluation have demonstrated myofiber disarray, but in the absence of significant hypertrophy.8
Idiopathic Endomyocardial Fibrosis
Clinical Findings
Idiopathic endomyocardial fibrosis is a disorder of the tropical and subtropical regions that is clinically characterized by restrictive cardiomyopathy. The eponym term “Davies disease” has been used because of descriptions of the disease by Davies in the 1950s and 1960s in Africa.11,12,13,14 It affects predominantly children and young adults.15 The disease is endemic in India and South America, as well as Africa.16 It is rare in the United States.
In contrast to eosinophilic endomyocarditis, most patients do not have peripheral eosinophilia. Ventriculograms demonstrate changes in ventricular morphology with restriction or obliteration in the inflow tract or apex, reduction in the diastolic ventricular dimension, endocardial irregularities, and, in severe cases, a tubular distortion of the ventricle due to obliteration of the inflow tract. Mitral and tricuspid regurgitation are typical. Magnetic resonance imaging findings include ventricular diastolic dysfunction, systolic dysfunction, and extensive subendocardial delayed contrast enhancement with atrial enlargement and organized ventricular thrombus.17 Surgical treatment includes valve replacement or repair, and resection of the endocardial fibrotic tissue.16 In instances, surgical treatment is curative. Even though bilateral disease is typical, there may be isolated left or right ventricular involvement.15
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree