Chapter 28
Restrictive Cardiomyopathy
1. What is the basic physiologic problem in restrictive cardiomyopathy?
2. What are the main causes of restrictive cardiomyopathy?
Approximately half the cases of restrictive cardiomyopathy have an identifiable cause. The most common identifiable cause is myocardial infiltration from amyloidosis. Other infiltrative diseases include sarcoidosis, Gaucher disease, and Hurler disease. Storage diseases include hemochromatosis, glycogen storage disease, and Fabry disease. Endomyocardial involvement from endomyocardial fibrosis, radiation, and anthracycline treatment can also lead to restrictive cardiomyopathy. Although restrictive cardiomyopathy is a rather uncommon cause of heart failure in North America and Europe, it is a common cause of heart failure and death in tropical regions, including parts of Africa, Central and South America, India, and other parts of Asia (where the incidence of endomyocardial fibrosis is relatively high). Causes of restrictive cardiomyopathy are summarized in Table 28-1.
TABLE 28-1
CLASSIFICATION OF TYPES OF RESTRICTIVE CARDIOMYOPATHY ACCORDING TO CAUSE
Infiltrative
Amyloidosis
Sarcoidosis
Gaucher disease
Hurler disease
Fatty infiltration
Storage Disease
Hemochromatosis
Fabry disease
Glycogen storage disease
Endomyocardial
Endomyocardial fibrosis
Radiation
Hypereosinophilic syndrome (Löffler disease)
Carcinoid syndrome
Myocardial Noninfiltrative
Idiopathic cardiomyopathy
Familial cardiomyopathy
Hypertrophic cardiomyopathy
Scleroderma
Modified from Kushwaha S, Fallon, JT, Fuster V: Restrictive cardiomyopathy, N Engl J Med 336:267-276, 1997.
3. What are the usual echocardiographic findings in restrictive cardiomyopathy?
Echocardiography usually demonstrates normal or near-normal systolic function, ventricles of normal or decreased volumes, normal or only minimally increased ventricular wall thickness, impaired ventricular relaxation and filling (diastolic dysfunction), and biatrial enlargement. As discussed in Question 4, these findings may be different in later stages of amyloidosis and certain other conditions (Fig. 28-1, A). On Doppler echocardiography, one observes accentuated early diastolic filling of the ventricles (prominent E wave), shortened deceleration time, and diminished atrial filling (diminutive A wave) resulting in a high E-to-A wave ratio on the mitral inflow velocities (see Fig. 28-1, B).
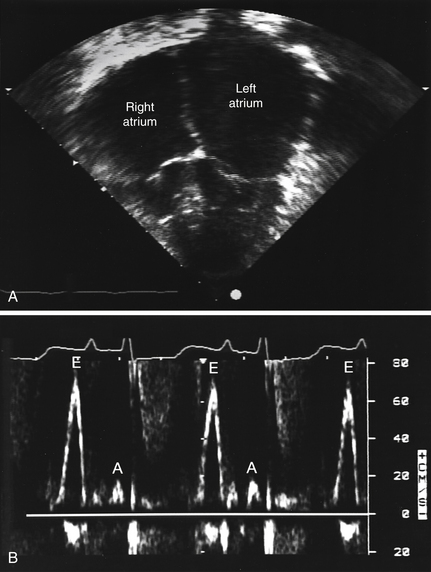
Figure 28-1 Echocardiogram of a patient with familial restrictive cardiomyopathy. A, Apical 4-chamber view with dilated atria and normal ventricles. B, Mitral inflow pattern showing augmented early filling E waves (E) with shortened deceleration time and attenuated A waves (A) with minimal respiratory variation in velocity. (Modified from Schwartz ML, Colan SD: Familial restrictive cardiomyopathy with skeletal abnormalities. Am J Cardiol 92:636-639, 2003.)
4. How does amyloidosis affect the heart?
As with other organs, in amyloidosis there may be protein deposition in myocardial tissue. The term amyloidosis was reportedly coined by Virchow and means “starchlike.” The affected myocardium is found to be firm, rubbery, and noncompliant. This protein deposition leads to restrictive physiology, as well as to eventual systolic heart dysfunction and possible conduction abnormalities. Patients are often extremely fluid sensitive, and management is complicated by having to walk a fine line between volume overload and inadequate preload. Patients with amyloidosis often manifest orthostatic hypotension and may develop additional conduction-related disorders. The prognosis is generally extremely poor.
5. How is cardiac amyloidosis diagnosed?
If the patient is not otherwise known to have amyloidosis, cardiac amyloidosis may be suggested by symptoms and signs of heart failure, an echocardiogram that demonstrates impaired filling and often thickened ventricular walls, a “sparkling” pattern on echocardiography (Fig. 28-2), and low voltage on the electrocardiogram (in spite of the thickened ventricular walls). Radionuclide imaging showing increased diffuse uptake of technetium-99m (99mTc) pyrophosphate and indium-111 (111In) antimyosin in cardiac amyloidosis can also be used to make the diagnosis. The diagnosis is usually confirmed, if necessary, by biopsy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


