Acute lower respiratory tract diseases
Bronchiolitis
Pneumonia
Pulmonary edema
Acute chest syndrome
Atelectasis
Avoidance of intubation or re-intubations
Do-Not-Intubate (DNI) or comfort-measures-only (CMO)
Immunocompromised status
Neuromuscular disorders
Neurological illnesses
Cystic fibrosis
Restrictive lung disease (e.g. severe scoliosis)
Postoperative respiratory insufficiency
Postextubation respiratory insufficiency
Aid to successful extubation
Overlap with invasive mechanical ventilation to facilitate early weaning and extubation
The successful use of NIV in parenchymal lung diseases has been well described for decades. NPV was the first modality of NIV used back in the 1930s throughout the 1950s during the polio epidemic, and then recently with more recent evidence to support its effectiveness in patient with bronchiolitis and other causes of parenchymal lung diseases like pneumonia [22]. NPPV, including CPAP and BiPAP, is an effective modality to support pediatric patients with mild and moderate acute respiratory insufficiency [18, 28, 42, 44–48] associated with bronchiolitis, pneumonia [49, 50], status asthmaticus [41], pulmonary edema, and atelectasis. In a large retrospective study by Ganu et al. including 520 children with bronchiolitis, 285 patients were supported with NIV. Of the NIV-supported patients, 237 (83.2%) needed only NIV and 48 (16.8%) failed and required intubation. Patients successfully supported by NIV had significantly shorter median length of stay compared to those requiring invasive ventilation and those who failed NIV (2.38 ± 2.43 vs. 5.19 ± 6.34 vs 8.41 ± 3.44 days, respectively; p < 0.001) [42].
In a prospective study, Munoz-Bonet et al. [50] reported using NIV in 47 episodes for 37 patients with acute hypoxic respiratory failure with an 80% success rate. NIV-failure was due to progression of respiratory failure and was observed between 3 and 87 hours (average 33.6 ± 29.6 hours) after initiation. Heart rate and PCO2 significantly improved after NIV implementation. Maximum mean airway pressure of 11.5 cmH2O and oxygen requirements more than 60% predicted NIV failure.
While its association with favorable outcomes makes the use of NIV an attractive alternative to IMV, appropriate patient selection is paramount because failure of NIV has been associated with higher morbidity and mortality in acute hypoxic respiratory failure and PARDS. Because of lack of strong consistent data, current consensus guidelines do not support the routine use of NIV in patients with moderate to severe PARDS [26]. Therefore, clinicians should be judicious with its use, selecting only patients in whom the most benefit is expected.
In some patient populations, the potential benefit of NIV may far outweigh the risk of initiation of IMV. NIV could be the only appropriate ventilation modality for terminally ill patients with Do-Not-Intubate (DNI) or comfort-measures-only (CMO) status in place. Implementing NIV in these circumstances could be needed to get through an acute illness or to provide comfort at the end of life [51].
In pediatric oncology and immunocompromised patients, the risk of mortality associated with IMV makes a trial of NIV warranted and desirable. While survival of this group of critically ill patients has improved, mortality associated with acute respiratory failure and PARDS remains high [52–54]. These patients continue to represent a challenging population in critical care units. It is estimated that about 40% of these patients requires intensive care admission throughout the disease course. Development of ARF/PARDS and associated complications with IMV are major determinants of poor outcomes. Recent advances in respiratory support, especially NIV, have allowed more options to support these patients early on while in the PICU.
NIV has been suggested as the first modality of respiratory support in mild and possibly moderate PARDS in immunocompromised patients [18, 26, 52, 53, 55–58]. In a retrospective Italian study, Piastra et al. [54] showed that NPPV use was feasible in immunocompromised/oncology patients with PARDS. Out of 23 immunocompromised children with PARDS requiring mechanical ventilation, 13 (56%) were successfully supported with NPPV. The NPPV-successful group had a shorter ICU and hospital stay, less hospital-acquired infections, and lower reported incidence of septic shock. In another retrospective study, Fuchs et al. [59] investigated the mortality rate and the clinical variable related to the use of NPPV in 41 immunocompromised children with ARF. Eleven were successfully supported with NIV, of which 8 had recurrence of respiratory insufficiency within 27 days. The study showed that lower FiO2, lower SpO2/FiO2 ratio, and bacterial septicemia were predictive of NIV success, while fungal septicemia and culture-negative acute respiratory insufficiency were predictive of NIV failure. In addition, the overall prognosis of ARF in immunocompromised children was independent of the NIV failure. In a more recent large retrospective cohort study, Pancera et al. reported a NIV success rate of 74.2% in 120 immunocompromised children with ARF. Solid tumors and cardiovascular dysfunction predicted NIV failure [60].
Weaning and early extubation is desired in patients with PARDS. NIV has been proposed to facilitate early weaning from IMV, most commonly by implementing NPPV preemptively in high-risk patients (e.g., neuromuscular illnesses) immediately after extubation, especially following pulmonary complications after major surgical procedures [61–63]. NPPV has been used to treat postextubation ARF in adults and pediatrics for decades to avoid reintubation with encouraging results.
Anecdotally, clinical practices suggest the use of NPPV concurrently with IMV to facilitate separation from the ventilator despite “higher ventilator settings” than the usual practice.
Contraindication of NPPV in PARDS
Respiratory arrest |
Cardiac arrest |
Hemodynamic instability (shock) |
Severe PARDS |
The need for immediate intubation |
Rapid progression of neuromuscular illness |
Rapid worsening of neurological status |
Inability to handle oropharyngeal secretions |
Impaired gag or cough reflex |
Recent esophageal or gastric surgery |
Uncooperative patient |
Severe agitation |
Facial trauma |
Basal skull fracture with CSF leak |
Facial burns |
Untreated pneumothorax |
Complications of NPPV
Inadequate gas exchange |
Pulmonary aspiration |
Gastric distention and perforation |
Pressure sores (face, nose) |
Eye injury and irritation/conjunctivitis |
Barotrauma (pneumothorax, pneumomediastinum) |
Agitation |
High-Flow Nasal Cannula
The use of HFNC in pediatric acute hypoxemic respiratory failure has increased over the last decade, with nearly one-quarter of all children admitted to the PICU receiving this form of respiratory support [5]. The popularity of HFNC is likely related to its ease of use, portability, patient tolerability, and success in treating perinatal lung disease and viral bronchiolitis [33, 64]. Clinicians may also choose to use HFNC in children at risk for development of PARDS, although there are limited data describing these patients [17].
HFNC likely improves work of breathing and gas exchange in hypoxemic respiratory failure via reduction in inspiratory resistance, washout of nasopharyngeal dead space with oxygen-rich gas, reduction of metabolic work with delivery of conditioned gas, improved mucociliary clearance, and application of nominal levels of positive pressure [35–37]. The HFNC system includes the following basic elements: (1) a source of pressurized and blended oxygen and air; (2) a water reservoir attached to a heated humidifier; (3) a heated circuit that maintains temperature and humidity of the gas; and (4) a nonocclusive cannula interface.
With initiation of HFNC, the clinician sets the gas temperature, the FiO2, and the flow rate. We recommend an initial gas temperature 1–2 °C below body temperature. The initial HFNC FiO2 should be chosen based on patient’s need and physiology, and adjusted to target a chosen oxygen saturation (SpO2). While there is no consensus regarding the ideal gas flow rate, there is evidence to support weight-based dosing [34]. Modest respiratory support is provided with flow rates between 0.5 and 1.0 L/kg/min, while increasing the flow to 1.5–2.0 L/kg/min may attenuate intrathoracic pressure swings to further reduce work of breathing [65]. Flows greater than 2 L/kg/min may not provide additional benefit [66].
Continuous Positive Airway Pressure (CPAP)
CPAP, similar to other forms of NIV, may be considered both for patients “at risk for PARDS” and in those with mild and moderate PARDS to avoid complications of IMV. However, intubation and mechanical ventilation should not be delayed and must be considered early in patients without signs of improvement or with worsening respiratory status within the first few hours of NIV initiation [26].
According to the most recent Pediatric Acute Lung Injury Conference Consensus (PALICC) recommendations, pediatric patients with ARF receiving nasal CPAP and requiring ≥40% FiO2 are considered “at risk of PARDS,” while ARF patients receiving full face-mask CPAP≥5 cm H2O with PaO2/FiO2 ratio (PF ratio) ≤300, or oxygen saturation/FiO2 ratio (SF ratio) ≤264, meet PARDS criteria.
CPAP refers to the application of a constant flow with resultant constant positive pressure throughout the respiratory cycle while the patient is spontaneously breathing. The use of CPAP to support children with ARF has been mainly described in patients with bronchiolitis and asthma [34, 41, 67, 68]. In a randomized controlled study including 142 infants with severe viral bronchiolitis, Milési et al. showed that CPAP is superior to HFNC with a relative risk of success of 1.63 (95% CI 1.02–2.63), higher with CPAP compared with HFNC. Failure occurred in 31% in the CPAP group and 50.7% in the HFNC group [34]. These results were similar to a previous single-center randomized trial in a similar patient population [67].
There are different types of interface used in NPPV: Nasal prongs, oronasal (full-face) mask, nasal mask, helmet, total face mask, and mouth piece. Oronasal is the most commonly used interface in acute settings. This can be effective in improving gas exchange and lung recruitment in PARDS. Gastric distention can be problomatic, so special attention should be taken while caring for these patients to avoid vomiting and minimize risk of aspiration [18, 39, 61, 69, 70].
CPAP of 6–12 cmH2O with FiO2 0.4–0.6 would be acceptable initial settings. FiO2 should be titrated to achieve SpO2 88 to ≤97%. Weaning of CPAP may be attempted once underlying pathology is resolving. CPAP can be trialed off when the patient has been stable at a level of 5–6 cm H2O with an FiO2 < 0.40.
CPAP mode is usually well tolerated in children [34, 48, 71], but it is not unusual that patients need sedation at initiation or throughout the implementation to facilitate patient-interface tolerance [72].
Bi-Level Positive Airway Pressure (BiPAP)
Among children with acute hypoxemic respiratory failure treated with NIV, only those receiving oronasal (full-face) mask CPAP or BiPAP are classified as meeting PARDS criteria [26]. Despite the PALICC recommending clinicians not use BiPAP in children with moderate and severe PARDS, the use of this mode of NIV continues to gain popularity [28, 29]. In a multicenter, prospective study including over 15,000 pediatric admissions, use of NIV, including BiPAP, was associated with decreased hospital length of stay and decreased mortality [6].
A well-fitted facial mask is essential for effective BiPAP use. Air-leaks around an ill-fitting mask will prevent generation of adequate mean airway pressure. Masks that are too tight, however, can lead to skin breakdown and pressure ulcers that preclude continued use. There are multiple patient interfaces available, with the oronasal (full-face) masks being most commonly used in the PICU. For most ventilators, the clinician will set up PEEP, inspiratory time, pressure support above the PEEP, a back-up mandatory respiratory rate, and FiO2.
With an appropriately fitting mask and patient-ventilator synchrony, BiPAP can effectively provide airway pressure, improve oxygenation, and unload fatigued respiratory muscles in children with acute respiratory failure. BiPAP is generally well tolerated, and its low-risk profile makes it an attractive first-line support therapy. Care should be taken, however, in patients with persistently low PF ratio, low SF ratio, and elevated respiratory rates, as these have all been associated with BiPAP failure [73–75].
Neurally Adjusted Ventilatory Assist (NAVA)
Neurally adjusted ventilatory assist (NAVA) is a relatively recent modality of mechanical ventilation. It is a pressure-assisted mode that utilizes the electrical activity of the diaphragm (EAdi) to trigger a spontaneous assisted breath and deliver inspiratory pressure in response to that activity. NAVA detects this electrical activity through eight electromyogram detectors located at the end of special nasogastric or orogastric tube. The distal end of this tube is usually placed at the end of the esophagus near the gastroesophageal junction where the trunk of the phrenic nerve meets the diaphragmatic muscle.
NAVA has been successfully used in acute respiratory failure in intubated children and adults on mechanical ventilation. NAVA has been shown to improve patient synchrony with the ventilator, decrease need for sedation, and possibly reduce PICU length of stay [76–83].
In a prospective randomized cross-over study, Vignaux et al. [84] reported improved patient-ventilator synchrony in infants and children with ARF receiving NIV while on NAVA. In a more recent prospective study, Baudin et al. [77] described the use of NIV-NAVA in 11 infants less than 6 months of age with ARF. The study showed that the asynchrony index was significantly lower in NAVA mode compared to pressure-assist control mode (3 ± 3% and 38 ± 21% respectively P < 0.0001). There were more ineffective breathing efforts in pressure control mode than NAVA as well (21.8 ± 16.5 vs. 0.54 ± 1.5 events/minute, respectively). More studies are needed to evaluate the use on NIV-NAVA in PARDS.
Negative Pressure Ventilation (NPV)
NPV was the first form of ventilator used to treat respiratory failure using the “iron lung.” Recently, there has been a renewed interest in the use of NPV in children with ARF. To date, there are few reports, mostly case series and case reports, describing the use of NPV in pediatric population with acute respiratory failure due to different etiologies [22, 85–93].
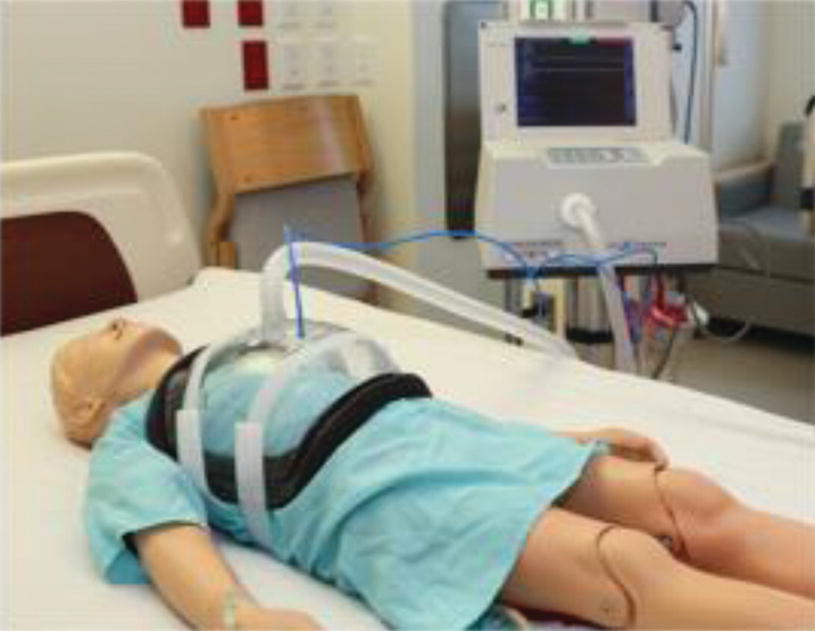
NPV cuirass ventilator
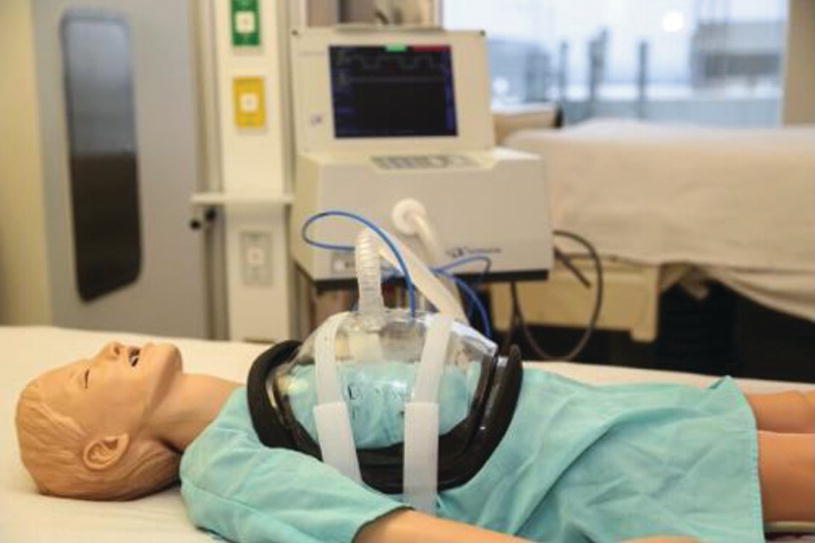
NPV cuirass ventilator
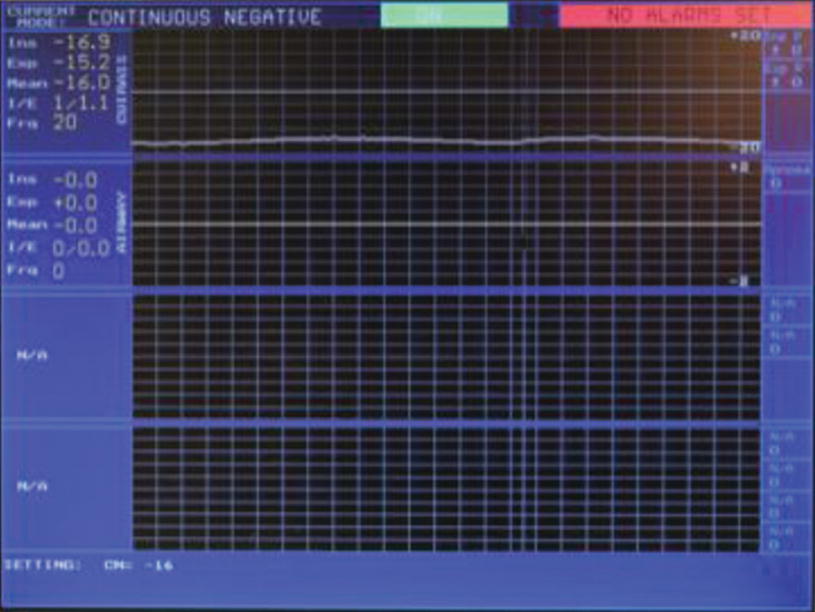
NPV cuirass ventilator: Screen shot, Continuous NEgative Pressure (CNEP) mode
In an animal study comparing NPV and PPV using surfactant-depleted rabbits, Grasso et al. [87] showed that NPV was associated with better gas exchange, greater lung perfusion, better lung expansion, and less lung injury.
Advantages, disadvantages, and complications of NPV
Advantages Avoidance of risks of positive pressure ventilation (e.g., barotrauma, decreased venous return) Comfort Ability to speak Access to oral and nasal secretions Less need for sedation Suitable for patients with facial trauma/burns Reduced risk of aspiration |
Disadvantages Cannot be used in patients over 170 kg Cuirass fitting can be a challenge in patients under 4 kg Requires a patent/viable airway |
Contraindications Burns on the chest wall or abdominal wall Thoracic and abdominal surgery Chest and abdominal trauma – flail chest Respiratory arrest Cardiac arrest Hemodynamic instability (shock) Severe PARDS Need for immediate intubation Rapid progression of neuromuscular illness Rapid worsening of neurological illness Inability to clear oropharyngeal secretions Impaired gag and cough reflex |
Similar to other modalities of NIV, NPV can be considered for “at risk for PARDS” patients as well as patients with mild PARDS. Further studies are needed to compare NIV and NPPV, to evaluate the role of NIV in mild-to-moderate PARDS, and to assess outcomes (e.g., rate of intubation, complications).
Patient Monitoring While Receiving NIV
Pediatric patients requiring NIV for acute respiratory failure should be admitted to the PICU. However, there is some evidence to suggest that HFNC, and possibly nasal CPAP, are safely delivered on a general inpatient ward, primarily in patients with viral bronchiolitis. Monitoring of heart rate, respiratory rate, continuous pulse oximetry (SpO2), and noninvasive blood pressure is necessary. Although the optimal fluid management strategy in these patients is yet to be defined, current consensus guidelines recommend judicious use of fluids to maintain appropriate intravascular volume. Hemodynamic monitoring during NIV in PARDS is important to appropriately guide the fluid management therapy and avoid fluid overload. Monitoring urine output, capillary refill, and peripheral pulses is recommended [26].
The Pediatric Acute Lung Injury Consensus Conference (PALICC) recommended that Oxygenation index (OI = FiO2 × Mean Airway Pressure × 100)/PaO2) is the preferred metric to define PARDS in patients supported by IMV, while PaO2/FiO2 ratio (PF ratio) is the primary metric to define PARDS in patients with NIV receiving CPAP or BiPAP with a minimal CPAP level of 5 cmH2O.
Oxygen Saturation Index (OSI = FiO2 × Mean Airway Pressure × 100)/SpO2) and Oxygen saturation/FiO2 ratio (SF ratio) are recommended to use for monitoring in cases where PaO2 value cannot be obtained. Oxygen supplementation must be titrated to achieve SpO2 88 to ≤97%. Children requiring full mask CPAP or BiPAP ≥5 cmH2O meet PALICC PARDS criteria when PF ratio ≤ 300, or Oxygen saturation/FiO2 ratio ≤ 264 [26]. There is no severity stratification of OI and OSI during noninvasive mechanical ventilation, but OI > 4 and OSI > 5 are considered abnormal.
Blood gas measurements (arterial, venous, capillary) add further information on gas exchange, help the critical care providers to better assess the clinical status, and provide guidance to escalate therapy as needed. There is no consistency in the literature to support the timing and frequency of sampling, but oxygenation indices and other gas exchange metrics should be evaluated at onset of PARDS, initiation of NIV support, within 24 hours of initiation, and serially at the discretion of the critical care providers determined by the patient’s clinical progression.
Special attention should be given to avoid nasal and facial pressure sores [94, 95]. Initially by avoiding fitting the interface too tightly, followed by implementing safety practices with frequent checking of the pressure areas, adjusting the interface accordingly, and by judicious use of gel pads and cushions to protect the skin.
Need for Sedation During NIV
To be physiologically beneficial, all forms of NIV require patient-interface tolerance and synchrony. While most patients tolerate treatment with nasal CPAP and HFNC, many younger children and infants may have difficulties in enduring face mask positive pressure [4] and NPV [22]. Agitation can precipitate patient-ventilator asynchrony, diminishing its effectiveness and leading to barotrauma. Pharmacologic sedatives and anxiolytics can be safe and effective, provided the patient anxiety is not due to impending respiratory failure requiring immediate invasive mechanical ventilation.
The ideal sedative should provide appropriate anxiolysis without affecting respiratory drive, airway tone, or hemodynamics. Midazolam has been used successfully in children with status asthmaticus [96], and infants with hypoxemic respiratory failure [97], although concerns regarding hemodynamic stability, airway tone, respiratory drive, and long-term neurologic morbidity may limit use.
Dexmedetomidine use has experienced an increase in popularity. A recent single-center study described 202 children with acute respiratory failure due predominantly to status asthmaticus and bronchiolitis treated with NIV (defined as CPAP, BiPAP, HFNC) and concurrent dexmedetomidine infusion. Most received dexmedetomidine as a single agent, with 83% of included patients achieving adequate sedation. The majority of patients did well, with 98% successfully weaned off NIV without need for intubation. However, clinically significant events included bradycardia (13%), hypotension (20%), and hypopnea (5%), while a one-month-old infant with bronchiolitis required CPR and vasoactive medications following apnea and bradycardic arrest during dexmedetomidine infusion. Dexmedetomidine is an alpha-2 adrenergic agonist with minimal effects on respiratory drive, thus making it an attractive sedative agent [98], but the negative effects on hemodynamics, including decreased catecholamine release [99], decreased cardiac index, bradycardia, and hypotension, should be carefully considered when initiating this medication. Risk of withdrawal must also be considered with dexmedetomidine use, [100, 101] although this is generally only of consequence following prolonged infusions [102].
Failure of Noninvasive Ventilation
Some children clearly require immediate intubation and IMV, and for these, NIV is contraindicated. Many children benefit from NIV and recover from their acute lung injury. There are concerns, however, that application of NIV may mask progressive worsening of respiratory failure, delay the timing of intubation, and increase the risk of associated complications, including death [103, 104]. Furthermore, the failure rate for NIV is variable, and likely dependent upon the underlying disease process and the chosen mode of ventilation.
Evidence in support of NIV, including reduced intubation rates, is best established in infants receiving CPAP or HFNC with bronchiolitis [33, 34, 105, 106]. Studies demonstrating successful treatment of bronchiolitis with either modality show improvements in respiratory effort as measured by vital signs, clinical respiratory scores, and gas exchange [34, 107–109]. Clear recommendations for use of NIV in other forms of pediatric acute hypoxemic respiratory embarrassment, including PARDS, are lacking [110]. For infants with bronchiolitis, failure rate with use of CPAP and HFNC can be as low as <3%, [34], but for children with PARDS, it is as high as 50% [2]. Understanding which patients are unlikely to be successful NIV candidates, and indicators of failure, are especially important as use of NIV in the pediatric population continues to increase [5, 28].
Many children with PARDS, those at risk for PARDS, or those with acute lung injury/acute hypoxemic respiratory failure ultimately require IMV despite initial treatment with NIV [17]. A recent international, multicenter, prospective observational study including pediatric patients with PARDS found that of the 708 included patients, 22.6% received NIV as first-line respiratory support, and half of these patients (n = 80) ultimately required IMV. These children who failed first-line therapy with NIV had higher rates of PICU mortality and 90-day mortality compared to those children successfully managed with NIV [2]. In this cohort, more severe hypoxemia at PARDS diagnosis was strongly associated with subsequent intubation.
In several single-center studies, NIV failure was associated with higher severity of illness at admission, number of organ failures, nonrespiratory primary diagnosis, higher oxygen requirement, and higher respiratory rate [73, 74, 111, 112].
Summary
With appropriate patient selection, NIV is a viable alternative to IMV in children with acute respiratory failure and mild PARDS. Despite the scarce data to support NIV use in moderate to severe PARDS, clinical evidence of its usefulness has led to its increasing use in critical care units over the last two decades. Close monitoring is important to assess disease progression, avoid delaying appropriate therapy, and reduce potential complications. Intubation should be considered in patients receiving NIV who fail to show clinical improvement or have signs and symptoms of worsening disease process within few hours of NIV implementation.

Full access? Get Clinical Tree


