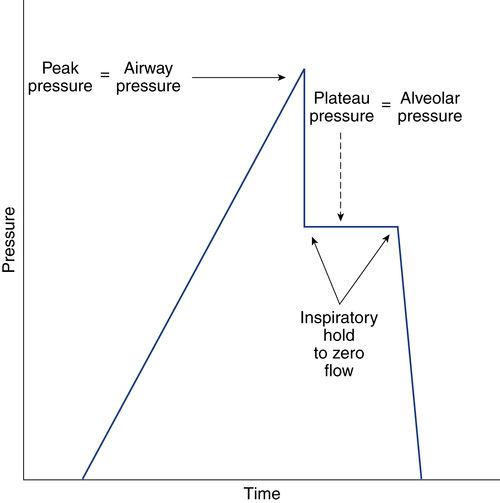
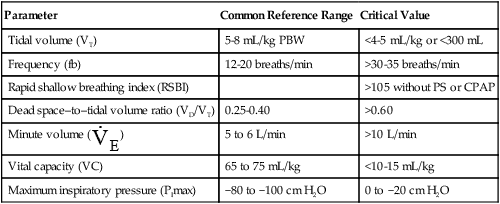
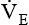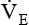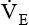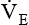After reading this chapter, you will be able to: 1. Identify the methods, normal values, and significance of measuring the following lung volumes in the intensive care unit: b. Rapid-shallow breathing index d. Functional residual capacity 2. Identify the methods, normal values, and significance of measuring the following airway pressures or related indices in the intensive care unit: f. Maximum inspiratory pressure 3. List the definition, methods of detection, and methods of minimizing auto-PEEP. 4. Describe the value of monitoring pressure, volume and flow waveforms, and pressure-volume curves in mechanically ventilated patients. 5. Describe the methods and significance of measuring the fraction of inspired oxygen and exhaled carbon dioxide in the intensive care unit. 6. List the components of oxygen transport and their significance. 7. List the components involved in the clinical evaluation of oxygenation and their significance. 8. Explain how the following parameters can be used to evaluate tissue oxygen delivery and utilization: a. Oxygen delivery and availability c. Mixed venous oxygen tension e. Arterial-to-mixed venous oxygen content difference h. Regional tissue oxygenation 9. Describe the value and limitations of pulse oximetry in monitoring oxygenation and oxygen delivery. 10. Identify the techniques for monitoring tissue oxygenation and utilization. arterial-to-alveolar tension ratio arterial-to-mixed venous oxygen content difference (C(a − auto–positive end-expiratory pressure (auto-PEEP) dead space–to–tidal volume ratio (VD/VT) functional residual capacity (FRC) intrapulmonary shunt ( maximum inspiratory pressure (PImax) mixed venous oxygen saturation (S mixed venous oxygen tension (P near infrared spectroscopy (NIRS) oxygen extraction ratio (C(a − oxyhemoglobin dissociation curve peak inspiratory pressure (PIP) rapid-shallow breathing index (RSBI) Monitoring has been defined as “repeated or continuous observations or measurements of the patient, his or her physiologic function, and the function of life support equipment, for the purpose of guiding management decisions, including when to make therapeutic interventions, and assessment of those interventions.”1 Arterial pressure of carbon dioxide (Paco2) is traditionally thought of as the standard for assessing ventilation (see Chapter 8). However, changes in the patient’s metabolism, lung mechanics, ventilatory efficiency, and equipment function will occur before changes are seen in the blood gases. It is therefore important to monitor the ventilatory parameters in addition to the blood gases. Table 14-1 provides a list of frequently used ventilatory parameters and their commonly cited reference ranges and critical values, with the critical value representing the threshold for insufficiency. In general, the respiratory therapist (RT) should not judge a patient’s status based on a single critical value, but instead should assess these parameters in combination with each other. TABLE 14-1 Common Parameters Used for Ventilatory Assessment CPAP, continuous positive airway pressure; PBW, predicted body weight; PS, pressure control. Ventilation is the process of moving gases between the atmosphere and the lung. These gases occupy spaces commonly called lung volumes. Although lung volumes have been described and their measurement discussed in Chapter 9, their importance to the critical care clinician is emphasized here. The reasons that lung volumes are important to the clinician are as follows: • They affect gas exchange in the lung. • They reflect changes in the patient’s clinical status (improvement or deterioration). • They indicate response to therapy. • They signal problems with the patient-ventilator interface (i.e., circuitry or ventilator settings). • They help ascertain the patient’s ability to breath spontaneously or be weaned from mechanical ventilation. • Patients being considered for mechanical ventilation • Patients receiving and being weaned from mechanical ventilation • Preoperative evaluation (especially upper abdominal and thoracic surgery) • Adult patients with respiratory rates greater than 30 breaths/minute • Patients with neuromuscular disease • Patients with central nervous system (CNS) depression • Patients with deteriorating blood gases • Patients receiving noninvasive positive-pressure ventilation Tidal volume (VT) is defined as the volume of air inspired or passively exhaled in a normal respiratory cycle. VT for a healthy person varies with each breath but usually ranges between 5 to 8 mL/kg of predicted body weight.2 VT has two components: alveolar volume (VA), or the portion of VT that effectively exchanges with alveolar-capillary blood, and dead space volume (VD), or the portion of VT that does not exchange with capillary blood. The common reference range cited for VD is 25% to 40% of the VT. The conductive airways and alveolar units that are ventilated but not perfused create the true or physiologic dead space. If the dead space exceeds 60% of the VT, the patient may need ventilator support to help with the associated increase in the work of breathing.2 In healthy, spontaneously breathing people, the VT occasionally increases to three or four times normal levels. These larger tidal breaths are known as sighs and normally occur about 6 to 10 times each hour. In acutely ill patients, there is often a loss of the sigh, and the size of the patient’s VT tends to diminish.3 A VT less than 5 mL/kg may indicate the onset of a respiratory problem.2 Impending respiratory failure causes VT to become more irregular.4 If shallow breathing without occasional sighing is maintained for prolonged periods, atelectasis and pneumonia may result, especially in patients breathing high oxygen concentrations or having compromised mucociliary clearance. Patients receiving continuous mechanical ventilation (CMV) are routinely ventilated with VT of 8 to 10 mL/kg, approximately two times the normal spontaneous VT. When normal spontaneous VT is used during CMV without positive end-expiratory pressure (PEEP), there is a reduction in functional residual capacity (FRC), an increase in intrapulmonary shunt, and a fall in partial pressure of arterial oxygen (Pao2). These potentially harmful conditions can be reversed in part or totally by increasing the VT or by applying PEEP.5 The use of higher VT ventilation can cause complications, particularly in patients with severe respiratory failure.6–9 Evidence exists that lung injury may occur with a high VT that increases alveolar pressures (plateau pressure) beyond 30 cm H2O.6,9 The use of high-VT ventilation may predispose patients to volutrauma, a lung injury that occurs from overdistention of the terminal respiratory units. Volutrauma often develops in nondependent lung regions and is a main reason why lung damage persists after recovery from severe protracted ARDS.10 To avoid this lung injury, patients at risk for developing ARDS should be ventilated with mechanical VT of 6 mL/kg or less at a higher frequency (breaths per minute) to maintain an acceptable acid-base balance (pH > 7.30).9 Unfortunately, patients receiving low VT (4 mL/kg) who have a strong respiratory drive may experience breath “stacking.”11 The stacking results from a patient trying to inhale a VT greater than set, decreasing their airway pressure and triggering an additional breath on top of the previous VT. When using a smaller VT, the application of PEEP maintains FRC and prevents the fall in Pao2. Although no single approach to setting PEEP has been adopted, a recent review recommends setting the PEEP level to that resulting in the best compliance and lowest driving pressure.6 where When a patient is ready to be weaned from mechanical ventilation, a spontaneous breathing trial (SBT) should be attempted. The patient should be monitored for gas exchange, respiratory distress, and hemodynamic stability during this trial. Box 14-1 outlines the criteria used to define SBT failure.12 VT also may decrease if the patient fatigues during the SBT, as indicated by breath volumes below 300 mL or less than 4 mL/kg.12 Usually more than one of these signs is present when a patient fails an SBT. RSBI values greater than 105 have been reported to be strong prognostic indicators of weaning failure.13 More predictive than a single measurement is the progressive change in RSBI. Patients who demonstrate a significant increase in their RSBI on ventilator removal are very likely to fail weaning.14,15 Serial measurements of the RSBI during a period of spontaneous breathing may more accurately predict the ability to be successfully weaned from mechanical ventilator support.15 However, early measures of RSBI during a spontaneous breathing trial appear to be of little value in predicting weaning outcomes in COPD patients.16 An increase in carbon dioxide production caused by an increased metabolism (as occurs with trauma or fever) or high carbohydrate loading accompanying parenteral feedings may result in an increased in For these reasons, Vital capacity (VC) is the maximum volume of gas that can be expired from the lungs following a maximal inspiration. The typical reference range for healthy subjects is 65 to 75 mL/kg of predicted body weight.2 The VC maneuver depends on the patient’s effort and position; the largest values usually are recorded with the patient in the upright position. As described in Chapter 9, VC can be measured as either a forced maneuver (FVC) or a slow maneuver (SVC). The SVC maneuver may be much easier for the patient to perform, especially if the patient is lethargic, medicated, or experiencing pain or has obstructive airway disease. Although many factors can contribute to a reduction in VC postoperatively, one of the most important is the incision site. Thoracic and abdominal surgeries produce a significant fall in VC postoperatively, and this reduction may persist for a week or more.17,18 Operative procedures below the umbilicus are associated with fewer pulmonary complications. A VC of 10 to 15 mL/kg is usually needed for effective deep breathing and coughing. Values below this range are usually associated with impending respiratory failure.2 Values greater than 15 mL/kg usually indicate adequate ventilatory reserve and the possibility of discontinuing CMV and extubation. FRC is the volume of gas remaining in the lungs at the end of a normal passive exhalation. It is rarely measured in the ICU. The FRC is continuously in contact with pulmonary capillary blood and undergoing gas exchange. It is composed of a combination of residual volume (RV) and expiratory reserve volume (ERV). Normally, FRC is about 40 mL/kg of predicted body weight, or about 35% to 40% of total lung capacity (TLC). FRC can vary from breath to breath by as much as 300 mL in healthy people.19 Changes in body position affect FRC, with the greatest values being recorded in the upright position.20 These changes in FRC between 30-degree Fowler and supine positions may not occur in overweight or obese patients.21 When alveolar volume falls, as with atelectasis, FRC is reduced, and there are regional changes in alveolar pressure-volume curves. Initially, as FRC decreases, dependent alveoli collapse and require higher distending pressures to inflate. Because the apical alveoli remain at least partially open, they are more compliant and require less pressure to inflate. Subsequently, during mechanical ventilation, the inspired volumes are preferentially distributed to the apices. This distribution of inspired volumes to nondependent, poorly perfused alveoli contributes to the abnormal gas exchange seen in patients with decreased FRC. Dependent atelectatic alveoli open throughout inspiration as alveolar pressure increases and collapse during expiration. Experimental evidence now demonstrates that repeated collapse and reinflation of alveoli leads to alveolar damage, capillary rupture, and considerable lung injury. The application of PEEP prevents alveolar collapse and may reduce the extent of acute lung injury.22–24 Therapeutic modalities, such as PEEP or continuous positive airway pressure (CPAP), increase FRC. This is the primary benefit to patients with atelectasis and refractory hypoxemia. It is important to monitor airway pressures for the following reasons: • To help determine the need for mechanical ventilation and the patient’s readiness for weaning • To help determine the site and thereby the cause of impedance to mechanical ventilation • To evaluate elastic recoil and compliance of the intact thorax • To help estimate the amount of positive airway pressure being transmitted to the heart and major vessels Peak inspiratory pressure (PIP) is the maximum pressure attained during the inspiratory phase of mechanical ventilation (Fig. 14-1). It reflects the amount of force needed to overcome opposition to airflow into the lungs. Causes of this opposition to flow include resistance generated by the ventilator circuit, the artificial airway (ET tube), and the patient’s airways and elastic recoil of the thoracic cage and the lungs. Sudden increases in PIP or peak airway pressure should alert the clinician to the possible presence of a patient-ventilator interface problem. Potential causes of an increase in peak pressure are listed in Box 14-2. An increase in PIP while the plateau pressure (explained later) remains unchanged suggests an increase in Raw. Common causes include bronchospasm, airway secretions, and mucous plugging. As a result of the relationship between PIP and Raw, monitoring the PIP provides valuable information about the bronchodilator-induced changes in lung function of the mechanically ventilated patient.25,26 It is important to note that whenever changes in the PIP are used for evaluating Raw, no changes in the inspiratory flow, flow pattern, or VT should be made. High PIP may cause barotrauma.23,27 However, evidence suggests that high peak alveolar pressures from overdistention lead to alveolar rupture, or volutrauma.23,28
Respiratory Monitoring in Critical Care
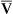 )o2)
)o2)
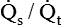 )
)
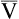 o2)
o2)
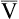 o2)
o2)
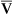 )o2/Cao2)
)o2/Cao2)
Ventilatory Assessment
Parameter
Common Reference Range
Critical Value
Tidal volume (VT)
5-8 mL/kg PBW
<4-5 mL/kg or <300 mL
Frequency (fb)
12-20 breaths/min
>30-35 breaths/min
Rapid shallow breathing index (RSBI)
>105 without PS or CPAP
Dead space–to–tidal volume ratio (VD/VT)
0.25-0.40
>0.60
Minute volume ( 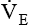 )
)
5 to 6 L/min
>10 L/min
Vital capacity (VC)
65 to 75 mL/kg
<10-15 mL/kg
Maximum inspiratory pressure (PImax)
−80 to −100 cm H2O
0 to −20 cm H2O
Lung Volumes and Flows
Why Monitor Lung Volumes?
Who Should Be Monitored for Lung Volumes?
What Do We Measure?
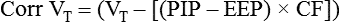
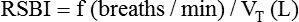
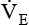 is the product of VT and respiratory rate or frequency and represents the total volume of gas inspired or exhaled by the patient in 1 minute. The average
is the product of VT and respiratory rate or frequency and represents the total volume of gas inspired or exhaled by the patient in 1 minute. The average 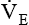 for a normal healthy adult is 5 to 6 L/minute.2 As with VT, approximately 25% to 40% of
for a normal healthy adult is 5 to 6 L/minute.2 As with VT, approximately 25% to 40% of 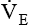 is dead space ventilation.
is dead space ventilation. 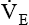 is often increased in the early stages of respiratory failure; it is not until later stages of failure that
is often increased in the early stages of respiratory failure; it is not until later stages of failure that 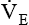 begins to fall.
begins to fall.
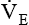 with a normal Paco2 (see Chapter 18). The elevated production of CO2 requires an increase in ventilation to maintain the Paco2 in normal range. Patients with varying metabolic rates should be ventilated with modes that allow them to set their own frequency of breathing and thereby vary
with a normal Paco2 (see Chapter 18). The elevated production of CO2 requires an increase in ventilation to maintain the Paco2 in normal range. Patients with varying metabolic rates should be ventilated with modes that allow them to set their own frequency of breathing and thereby vary 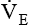 as needed to maintain a normal Paco2.
as needed to maintain a normal Paco2.
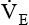 should be monitored frequently before and during weaning. A sudden rise or drop in
should be monitored frequently before and during weaning. A sudden rise or drop in 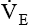 should be investigated because both may signal ventilatory failure. If a
should be investigated because both may signal ventilatory failure. If a 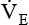 greater than 10 L/minute is needed for a mechanically ventilated patient to maintain a normal Paco2, weaning is not likely to be successful. The elevated
greater than 10 L/minute is needed for a mechanically ventilated patient to maintain a normal Paco2, weaning is not likely to be successful. The elevated 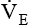 indicates that the patient’s respiratory muscles will probably fatigue when the mechanical ventilation is discontinued. Compared with the RSBI, however, a patient’s spontaneous
indicates that the patient’s respiratory muscles will probably fatigue when the mechanical ventilation is discontinued. Compared with the RSBI, however, a patient’s spontaneous 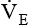 is a much less reliable predictor of weaning success.12,13
is a much less reliable predictor of weaning success.12,13
Airway Pressures
Peak Pressure
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Respiratory Monitoring in Critical Care