10 OBJECTIVES
Respiratory Infections
 Describe the major defensive mechanisms used by the host to remain free of respiratory tract infections.
Describe the major defensive mechanisms used by the host to remain free of respiratory tract infections.
 Recognize important pathogens in lower respiratory tract infections.
Recognize important pathogens in lower respiratory tract infections.
 Identify various risk factors for and clinical features of lower respiratory tract infections.
Identify various risk factors for and clinical features of lower respiratory tract infections.
 Understand the principles of management for these infections.
Understand the principles of management for these infections.
GENERAL CONSIDERATIONS
The respiratory tract is constantly exposed to a vast number of potentially harmful agents. As efficient as our pulmonary clearance mechanisms are, infectious agents still do deposit in the airways. Thus, in spite of the availability of potent antibiotics and great advances in supportive care, respiratory tract infections remain a major cause of disease, death, and health care costs. Pneumonia is the seventh leading cause of death in the United States and the most common cause of death due to an infection.
PATHOGENESIS: LUNG HOST DEFENSE MECHANISMS
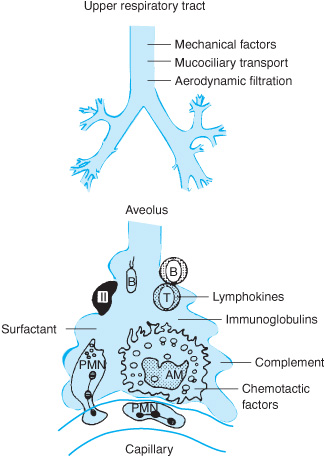
The defense system is distributed throughout the respiratory tract, from the nares to the alveolar surface (Table 10–1 and Figure 10–1). Infectious agents may gain access to the tract through inhalation of airborne droplets or aspiration of oral secretions. Smaller droplets (approximately 1–5 μm in size) remain airborne for long periods and may penetrate to the alveoli or be exhaled. Larger particles cannot remain airborne or are intercepted by anatomic barriers such as the nasal hair and turbinates, the epiglottis, and the larynx. The multiple branches of the tracheobronchial tree also serve a filtration function by causing deflection of the air stream, inducing airborne particles to impact and deposit on mucosal surfaces. The epithelium of the upper airway consists primarily of ciliated columnar epithelium covered by a mucus blanket. The cilia beat back and forth in a highly coordinated manner and interact with the mucus blanket to eventually deliver trapped particles to the oropharynx (the “mucociliary escalator”), where they are coughed out or swallowed. In distal bronchioles and alveoli, other factors are brought to bear: surfactant, iron-containing proteins, such as transferrin, IgG opsonins, and complement pathway activation are other defenses that serve as barriers against inhaled particles or microorganisms.
Table 10–1. Lung host defenses
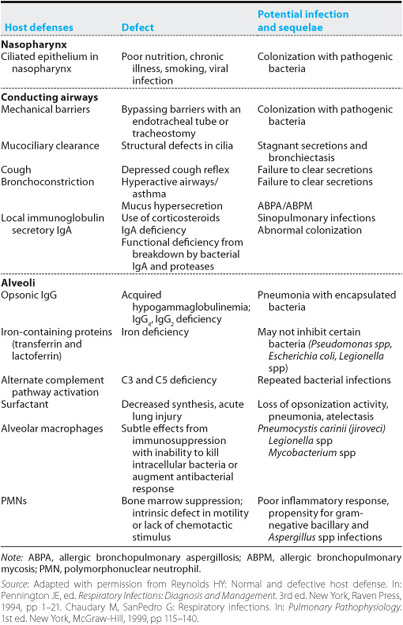
Figure 10–1. Representation of the defense mechanisms of the lung. (Reproduced with permission from Reynolds HY: Pulmonary defense mechanisms against infection. In: Fishman AP, ed. Pulmonary Diseases and Disorders. 2nd ed. New York, McGraw-Hill, 1988, pp 265–274.)
Alveolar macrophages are the principal phagocytic cells of innate immunity in the distal air spaces. They are ultimately responsible for engulfing and destroying material that evades upper airway clearance. The macrophage may present antigens to other effector cells through a series of complex interactions involving mediators also known as cytokines, initiating a specific adaptive immune response. Assisting the macrophages are other components of the humoral immune system and various lipoproteins and glycoproteins represented in the alveolar lining fluid. Should these local defenses prove inadequate, an inflammatory reaction can be initiated, unleashing potent polymorphonuclear neutrophils, complement factors, vasomediators, and other humoral immune elements from systemic sources. These mechanisms are highly efficient, rendering the lower respiratory tract sterile under normal conditions. The naso- and oropharynx are densely colonized by a complex mixture of aerobic, anaerobic, and microaerophilic bacteria. Normal oral flora may include Streptococcus spp including S pneumoniae, Neisseria spp, Candida spp, Fusobacterium spp, anaerobic streptococci, and certain Bacteroides spp. These organisms serve a protective function by competing with bacteria for adherence sites.
 Normally sterile respiratory tract sites include the paranasal sinuses and the lower tract (arbitrarily defined as that portion of the tract distal to the vocal cords). Haemophilus influenzae and Haemophilus parainfluenzae are commonly recovered from tracheobronchial secretions of smokers, even when there is no evidence of acute infection. In patients with chronic bronchitis, tracheobronchial colonization is common and may include H influenzae, H parainfluenzae, Moraxella catarrhalis, S pneumoniae, and occasionally gram-negative bacteria. Colonization with the last becomes particularly common in patients with ciliary dysfunction (including cystic fibrosis and bronchiectasis), patients on corticosteroid therapy, and those with immunodeficiency, prior antibiotic therapy, previous viral infection, malnutrition, or endotracheal intubation.
Normally sterile respiratory tract sites include the paranasal sinuses and the lower tract (arbitrarily defined as that portion of the tract distal to the vocal cords). Haemophilus influenzae and Haemophilus parainfluenzae are commonly recovered from tracheobronchial secretions of smokers, even when there is no evidence of acute infection. In patients with chronic bronchitis, tracheobronchial colonization is common and may include H influenzae, H parainfluenzae, Moraxella catarrhalis, S pneumoniae, and occasionally gram-negative bacteria. Colonization with the last becomes particularly common in patients with ciliary dysfunction (including cystic fibrosis and bronchiectasis), patients on corticosteroid therapy, and those with immunodeficiency, prior antibiotic therapy, previous viral infection, malnutrition, or endotracheal intubation.
Thus, normal hosts with their normal flora intact are resistant to colonization by many pathogenic bacteria, even when exposed to large quantities of the organisms. In contrast, ill individuals may have their indigenous bacterial flora replaced by the more virulent pathogens. The likelihood that a given individual will have upper airway colonization by these pathogenic bacteria is directly related to the severity and duration of illness. In patients with altered consciousness with swallowing difficulty and impaired host defenses, infectious secretions may be aspirated into the airways and cause disease. Risk factors for upper airway colonization include alcoholism, endotracheal intubation, neutropenia, prior antibiotic use, azotemia, coma, hypotension, smoking, surgery, prior viral illness, immuno-suppression, and malnutrition.
The humoral immune system also plays an important role in keeping the airways clear of potential pathogens. The immunoglobulin IgA, which accounts for up to 10% of the total protein in nasal secretions, has specific antibacterial and antiviral activity. Patients with selective IgA deficiency are at risk for recurrent upper respiratory tract infections and gram-negative lower respiratory tract infections. IgG is the predominant immunoglobulin in lower airway secretions and functions as an opsonin there.
Several kinds of infectious agents are responsible for respiratory disease in humans (Tables 10–2 & 10–3).
BACTERIAL PNEUMONIA
Pneumonia, or infection of the lung parenchyma, occurs in as many as 6 million Americans annually. It can be caused by organisms acquired in the community or from the hospital. The annual incidence of community-acquired pneumonia (CAP) in the United States is estimated to range from 2 to 4 million cases. Approximately 900,000 of these individuals require hospitalization, accounting for 3% of all patients hospitalized. Mortality among hospitalized patients with CAP is reported to be between 10% and 15% but approaches 35% among those treated in intensive care units (ICUs). However, the vast majority of CAP patients are treated as outpatients. While mortality for this group is low (less than 5%), CAP is estimated to result in 53 million days of reduced productivity. The incidence of CAP and its course vary within the population. It is most common at the extremes of life, among the very young and the very old, and during the winter months. The risk of death increases with age, and when there is significant coexisting illness (eg, chronic obstructive pulmonary disease, renal failure, congestive heart failure, or immunosuppression). The severity and modality of illness is also related to the virulence of the offending pathogen (eg, resistant gram-negative organisms or Legionella spp).
Table 10–2. Common pathogens for upper respiratory tract infections
Pharyngitis | Group A streptococci |
Laryngitis | Viruses |
Common cold | Rhinovirus |
Sinusitis | Haemophilus influenzae |
Epiglottitis | H influenzae |
Croup | Parainfluenza virus |
| Respiratory syncytial virus |
| Adenovirus |
| Mycoplasma pneumoniae |
Source: Reproduced with permission from Niederman SN, Sarosi GA: Respiratory tract infections. In: George RB, Light RW, Matthay MA, et al, eds. Chest Medicine: Essentials of Pulmonary & Critical Care Medicine. Baltimore, Williams & Wilkins, 1995, pp 377–429.
Hospitalized patients are exposed to a different set of bacterial flora. Many patients receive ventilator support. Hospital-acquired (or nosocomial) pneumonia (HAP), or health care–associated pneumonia (HCAP) or ventilator-associated pneumonia (VAP), is often due to resistant pathogens and has a high incidence of mortality. Pneumonia is the second most common cause of nosocomial infection, but the first leading cause of death in hospitalized adult patients in the ICU. The estimated annual incidence of HAP in the United States ranges from 130,000 to 300,000 cases. HAP also causes higher hospital costs and longer hospital stays. The mortality attributable to HAP is estimated at 27% to 33%. In patients receiving mechanical ventilation, the mortality rates may be as high as 70%. Several factors have been associated with a greater mortality rate, including Staphylococcus aureus and Pseudomonas aeruginosa as pathogens, severity of underlying illness, inappropriate antibiotic therapy, age, shock, prior antibiotic therapy, and duration of prior hospitalization and drug resistance strains.
Table 10–3. Common pathogens for lower respiratory tract infections
Bronchitis | Haemophilus influenzae |
| Streptococcus pneumoniae |
| Moraxella catarrhalis |
| Mycoplasma pneumoniae |
| Adenoviruses |
| Influenza |
| Rhinovirus |
| Respiratory syncytial virus |
Bronchiolitis | Respiratory syncytial virus |
| Parainfluenza virus |
| Adenoviruses |
| Rhinovirus |
Community-acquired pneumonia (CAP) | Streptococcus pneumoniae |
| Legionella pneumophila |
| M pneumoniae |
| H influenzae |
| Anaerobes |
| Staphylococcus aureus |
| Enteric gram-negative aerobes |
| Influenza virus |
| Respiratory syncytial virus |
| Adenovirus |
| Chlamydia pneumoniae |
| Pneumocystis carinii (jiroveci) |
Bronchiectasis | Pseudomonas aeruginosa |
| S aureus |
| Mucoid Escherichia coli |
| H influenzae |
Source: Reproduced with permission from Niederman SN, Sarosi GA: Respiratory tract infections. In: George RB, Light RW, Matthay MA, et al, eds. Chest Medicine: Essentials of Pulmonary & Critical Care Medicine. Baltimore, Williams & Wilkins, 1995, pp 377–429.
Pathophysiology
The onset of infection and proliferation of microorganisms within the alveolar space elicits an acute inflammatory response. Local effects of the cellular influx, cytokine production, and increased alveolar capillary permeability results in impaired ventilation and decreased lung compliance. Major ventilation-perfusion mismatch occurs with increased right-to-left shunting as consolidation progresses. Hypoxia, seen in severe pneumonia, produces pulmonary vasoconstriction which often attenuates the right-to-left shunt.
The reduced pulmonary compliance increases the work of breathing and with associated inflammation produces dyspnea. The local inflammatory response results in the production of a number of cytokines that migrate out of the lung and produce fever, leukocytosis, and a number of metabolic and vascular changes. Severe pneumonia may result in bacteremia, systemic inflammatory response syndrome (SIRS), septic shock, and multiorgan dysfunction syndrome. The most common cause of acute respiratory distress syndrome (ARDS) is sepsis secondary to pneumonia (see Chapter 13).
Clinical Implications
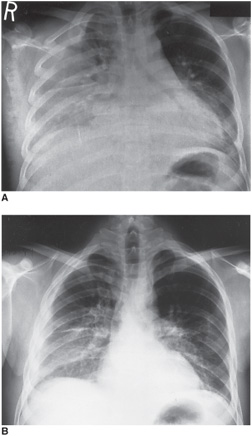
Two thirds of patients with CAP have a low mortality risk and could be managed on an outpatient basis (Table 10–4). The clinical presentation of individuals with bacterial pneumonia has traditionally been divided into typical and atypical pneumonia but recent evidence suggests that the diagnostic utility of this classification is doubtful (Figure 10–2A and B).
Management Principles
Management of individual cases of pneumonia is usually determined by the agent or agents suspected of causing the pneumonia. However, the spectrum of potential pathogens responsible for pneumonia is large, and use of traditional clinical, radiographic, and laboratory features or diagnostic tests is limited by problems with sensitivity and specificity, side effects, and expense. Even when diagnostic testing is used, most patients initially receive an empiric regimen and there are a number of guidelines for empiric therapy. Any delay in administration of an appropriate antibiotic can be associated with worse outcome, and a goal for therapy is to begin antibiotics within 4 hours of presentation (Table 10–5).
Table 10–4. Empiric outpatient treatment regimens for community-acquired pneumonia
Patients < 65 years of age without coexisting disease: |
Antimicrobial therapy: an oral macrolide or oral doxycycline |
Patients > 65 years of age and/or coexisting disease |
Antimicrobial therapy: second-generation cephalosporin, a β-lactam/β-lactamase inhibitor combination, or trimethoprim-sulfamethoxazole with or without a macrolide or new-generation fluoroquinolone |
Note: Coexisting diseases include diabetes mellitus, congestive heart failure, and chronic renal and hepatic insufficiency.
Source: Adapted with permission from Niederman MS, Bass JB, Campbell GD, et al. Guidelines for the initial management of adults with community-acquired pneumonia: Diagnosis, assessment of severity, and initial antimicrobial therapy. American Thoracic Society. Medical Section of the American Lung Association. Am Rev Respir Dis. 1993;148:1418.
Figure 10–2. A series of films from a patient with acute bacterial pneumonia. A.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree