Fig. 11.1
Kaplan–Meier survival curves showing percentage survival of ventilated versus non-ventilated patients 1967–2002 [7]
In ALS, NIV improves survival compared to patients who declined or were intolerant of treatment [26,27] and randomized [6] controls. The RCT showed a survival advantage of 7 months for patients with good bulbar function (Fig.11.2); of note, patients were enrolled regardless of their views on life prolonging therapy or social situation and those with more rapid progression were more likely to meet criteria for randomization within the timeframe of the study. In non-randomized studies, median survival from initiation of NIV of 14.2–18 months has been reported [11,27,28] and may be a better estimate of survival in well-motivated patients. In both the RCT of long-term NIV [6] and a prospective study of the use of NIV and cough assist during episodes of acute decompensation [29], a survival advantage was seen only in patients without severe bulbar impairment. However, there is evidence that patients with severe bulbar impairment who tolerate NIV survive longer than those who do not [26]. In a separate study, compared to historical controls, patients with severe bulbar impairment showed a survival advantage if they were hypercapnic, but not normocapnic, at the time of initiation [5]. This is biologically plausible; in such patients with poor airway protection, NIV may increase the risk of respiratory tract infections, but once daytime hypercapnia ensues, this risk may be outweighed by the benefits of ventilatory support. Compared to NIV, tracheostomy ventilation (TV) offers potentially longer survival, particularly if bulbar function is poor [30], but home care is less feasible.
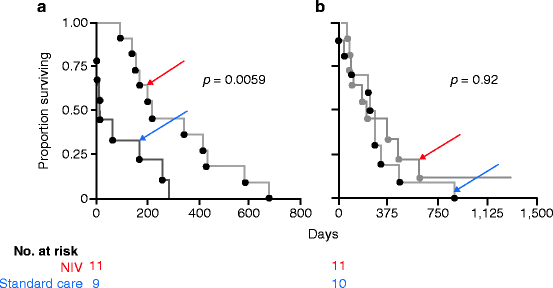

Fig. 11.2
Survival from randomization in a randomized controlled trial of non-invasive ventilation (red) compared to standard care (blue) in patients with amyotrophic lateral sclerosis and (a) normal or only moderately impaired bulbar function and (b) severe bulbar impairment [6]
The most important predictors of survival on NIV are adherence to treatment [11,28] and bulbar function [11]. Good nutritional status [28], younger age, and good upper limb function [11] are also associated with a better outcome. In ALS, about 5 % of patients suffer from fronto-temporal dementia, which is associated with poor adherence to NIV and gastrostomy feeding, older age, and shorter survival.
Symptoms and Quality of Life (QoL)
Of 59 patients with NMD who were switched from TV to NIV, all expressed a preference for NIV, whilst 35 patients switched from NIV to TV expressed a significant preference for NIV for convenience, effect on speech, appearance, comfort and general acceptability, and equal preference for sleep, swallowing, and security [31].
In DMD, most patients on long-term ventilation are satisfied with their QoL. Whilst physical function is limited, aspects of QoL reflecting sleep-related symptoms, emotional and social function, and mental health scores are nearly normal and similar to patients not requiring ventilatory support [32].
In ALS, NIV improves sleep-related symptoms, social and emotional function, mental health, and general wellbeing. After correcting for side effects related to NIV, overall QoL and, in particular, sleep-related symptoms show a substantial improvement [6,9]. Compared to patients with severe bulbar impairment, those with good bulbar function show larger improvements in symptom scores and QoL [6]. Most patients receiving NIV are cared for in their own home [33]. Compared to the carers of patients with similar physical limitation, but without respiratory impairment, carers of patients receiving NIV report similar QoL and strain [9]. Of interest, carers tend to significantly under-estimate the QoL experienced by the patient, whilst the patient tends to slightly over-estimate the QoL of their carer. Among patients receiving TV, the level of care and supervision required is much greater than that associated with NIV; consequently, home care is less feasible. The QoL of patients receiving TV in their own home is better than those in institutional care and similar to patients receiving NIV [33], but this is achieved at the expense of the QoL and burden experienced by their carers [30].
Patients may be afraid they may become trapped on a ventilator or, alternatively, will die a distressing death from suffocation. The latter concern was identified as one of the most important factors associated with the high rate of assisted suicide in patients with ALS in the Netherlands (ALS 20 %, cancer 5 %, and heart failure 0.5 %) [34]. It is important to reassure patients that if they elect to commence NIV, they will not become trapped on ventilation but rather this can be discontinued and effective alternative palliation provided if that is their wish. Symptoms such as breathlessness can be controlled and the reality is that most patients succumb peacefully to carbon dioxide narcosis. Palliation with opioids, sedatives, and oxygen therapy should not be commenced without first considering these issues and the role of ventilatory support.
Indications for NIV
In DMD, the results of two trials assessing early intervention (before daytime hypercapnia has ensued) showed conflicting results; the possible reasons for this are discussed above. In ALS, early initiation of NIV in patients with physiological impairment but no, or at most mild, symptoms is associated with higher overall NIV referral rates [35]. However, compared to standard criteria, adherence to NIV may be worse [11] and it is unclear whether early initiation confers an additional survival benefit; in patients with severe bulbar impairment the converse may be true [5]. A non-randomized trial reported that, compared to standard criteria for NIV, early intervention (VC ≥ 65 % predicted) was associated with longer survival [36]. However, the interval between disease onset and initiation of NIV was similar in both arms, suggesting that the rate of progression was slower in the early intervention arm, which may, at least in part, explain the apparent survival advantage. The optimal timing of initiation of NIV in ALS in patients with and without severe bulbar impairment warrants further study. Currently recommended criteria for initiation of NIV in DMD and ALS [37] are shown in Boxes11.1and11.2, respectively.
Box 11.1: Criteria for NIV in DMD
1.
Signs or symptoms of hypoventilation (patients with (F)VC < 30 % predicted are at especially high risk)a
2.
Baseline SpO2 < 95 % and/or blood or end-tidal PaCO2 > 6 kPa (45 mmHg)a
3.
An apnea-hypopnea index > 10/h on polysomnography or four or more episodes of SpO2 < 92 % or drops in SpO2of at least 4 %/h of sleepa
4.
Nocturnal hypoventilation: PTCCO2 > 6.5 kPa (48.8 mmHg)b
aBushby et al. [38]
bWard et al. [18]
Box 11.2a: Criteria for NIV in ALS Without Severe Bulbar Impairment
1.
(F)VC < 50 % of predicted
2.
(F)VC < 80 % of predicted plus any symptoms or signs of respiratory impairment
3.
SNIP or MIP < 40 cmH2O
4.
SNIP or MIP < 65 cmH2O for men or 55 cmH2O for women plus any symptoms or signs of respiratory impairment
5.
Rate of decrease of SNIP or MIP of more than 10 cmH2O per 3 months
6.
Nocturnal hypoventilation
(a)
SpO2 < 90 % for > 5 % of the night
(b)
PTCCO2 > 6.5 kPa (48.8 mmHg)
7.
Daytime hypercapnia (urgent referral)
Box 11.2b: Criteria for NIV in ALS with Severe Bulbar Impairment
1.
Daytime hypercapnia (PaCO2 > 6 kPa (45 mmHg))
2.
Nocturnal hypoventilation
(a)
SpO2 < 90 % for > 5 % of the night
(b)
PTCCO2 > 6.5 kPa (48.8 mmHg)
Abbreviations: (F)VC(forced) vital capacity,SpO 2oxygen saturation measured by pulse oximeter,PaCO 2partial pressure of carbon dioxide,P TC CO 2transcutaneous PaCO2, SNIPsniff nasal inspiratory pressure,MIPmaximum inspiratory pressure
Timing of Gastrostomy
Aspiration due to bulbar impairment, breathlessness during eating due to respiratory muscle weakness, and difficulty eating independently due to limb weakness may contribute to poor nutrition and weight loss. In the early stages, safe swallowing techniques, thickening of fluids and nutritional supplements may suffice. Placement of a gastrostomy tube improves survival [39] and should be considered if, despite these measures, the patient is failing to maintain adequate intake, has difficulty swallowing, and eating is tiring and/or has an unsafe swallow.
Percutaneous endoscopic gastrostomy (PEG) tube placement under conscious sedation is associated with a high complication rate in patients with VC < 50 % [39,40]. In patients with a rapid decline in respiratory function, early placement should be considered. In those with established severe respiratory muscle weakness (VC < 50 %, PImax/SNIP < 40 % predicted/40 cmH2O, daytime or nocturnal hypercapnia), radiologically guided insertion of a gastrostomy (RIG) tube may be a safer option; sedation is not required, the patient does not have to lie completely flat, and it is easier to provide support with NIV [41]. Alternatively, PEG insertion may be performed either with NIV support (with increased pressure support and FiO2to overcome airleak) or intubation and ventilation, followed by extubation onto NIV. Of importance, complications include diaphragmatic splinting and pneumonia, and most deaths occur after, not during, the procedure [40,41]; post-procedure respiratory care is important, including volume recruitment, cough assist, and, if required, NIV.
Acute Decompensation
Patients with NMD may present in extremis with acute, or acute on chronic, respiratory failure. Such episodes may be triggered by posture, injudicious use of uncontrolled oxygen therapy [42] or sedatives, mucus plugging, episodes of aspiration, respiratory tract infections, or pneumonia [29]. Of note, even upper respiratory tract infections are associated with a decline in respiratory muscle function. Whilst decompensation may be triggered by a reversible precipitant, most patients also have severe respiratory muscle weakness and, if they survive, will require long-term ventilatory support, particularly if the underlying condition is ALS or DMD [29] rather than a more slowly progressive disorder [43]. Consequently, when deciding whether or not acute ventilatory support is appropriate, and if so, how this should be provided, all factors influencing the outcome of both acute and long-term support, and the acceptability of this to the patient, should be borne in mind. The decision to commence ventilatory support may have to be made in an emergency, often by clinicians with limited knowledge of the underlying NMD; where possible it is preferable to discuss such issues with patients in advance and clarify their views. Structured respiratory care, including regular monitoring of respiratory function, reduces the need for emergency initiation of ventilation [5] but this is not entirely avoidable; unfortunately some patients present in acute respiratory failure before a diagnosis has been made.
In patients without severe bulbar impairment, compared to invasive ventilation, non-invasive ventilation reduces length of stay in intensive care and complications such as ventilator-associated pneumonia and may improve survival [29,44]. Compared to intubation, communication is better preserved using non-invasive support, facilitating subsequent discussions with the patient about their care, including long-term support. In addition to providing ventilatory support, it is also important to assist clearance of secretions [29,44]. Use of MI-E devices achieves better secretion clearance than deep suctioning and is preferable to mini-tracheostomy. Complications such as surgical emphysema are avoided and insufflation helps volume recruitment. Mucolytics, such as nebulized N-acetylcysteine 1–2 g, can be helpful. If a patient has been intubated to assist transfer between units, they can be extubated onto NIV. Those who have been inappropriately tracheostomized can also be converted to NIV once they are clinically stable [31]. If bulbar function is severely impaired, the patient is unlikely to survive the acute episode using non-invasive ventilation and cough assist alone [29]. If long-term TV is acceptable to the patient, they should be offered invasive ventilation; otherwise alternative palliation may be more appropriate.
Current Use of NIV
There has been a progressive increase in the proportion of patients with NMD treated with NIV over the last 10–15 years [4,7,35] A recent UK survey of respiratory care in ALS [35] showed that, compared to 2000, the proportions of patients referred for, and successfully established on, NIV have increased 2.6 and 3.4 fold, respectively. The most common deterrents to NIV referral were cognitive impairment, social isolation, rapid disease progression, and severe bulbar impairment. The neurologists who referred the most patients were more likely to monitor respiratory function regularly, measure respiratory pressures and arterial blood gases, consider early intervention, and rely on a combination of symptoms and physiological impairment to guide referral, reflecting the UK National Institute of Clinical Excellence (NICE) guidelines [37]. Wider application of NICE guidelines is likely to reduce variation in respiratory care and further improve the assessment of patients and use of NIV.
Recognizing the Terminal Phase in Progressive Neuromuscular Disease
In most neuromuscular conditions, currently available therapies have either no or only limited effect on the underlying disease process. Supportive treatments such as NIV and gastrostomy feeding improve survival in appropriately selected patients, but do not prevent progressive disability. The primary aim of most treatments is palliative; to optimize symptom control and improve quality of life.
In patients with progressive NMD, respiratory failure is the most common cause of death. However, as discussed above, symptoms are often only recognized at a late stage. Consequently, the degree of impairment in, and rate of decline of, respiratory function is key to anticipating the end stage of the condition [45]. Both within and between different NMDs, there is substantial variation in the rate of decline in respiratory muscle function, although within an individual patient the trajectory tends to be more consistent. Whilst there is often a gradual deterioration and a worsening of symptoms over the final few weeks and months of life, some patients deteriorate very quickly and die suddenly or within a few days [46]. Identifying when someone with a neuromuscular condition may be approaching the end of life can be very challenging. However, there are several indicators that may suggest the disease is progressing and trigger end of life discussions and planning (see Box11.3).
Regular assessment, tailored to the individual patient, is essential to ensure early recognition of the onset of the terminal phase of the condition. Clearly, there may be occasions when there is a reversible component to an observed deterioration in a person’s condition, which may respond to appropriate treatment if recognized. This may include inter-current infections, an underlying psychological condition such as depression, or an adverse response to medication changes or increases. Furthermore, despite disease progression, survival may be extended by non-invasive and tracheostomy ventilation and gastrostomy feeding. However, not all patients desire treatment measures that may extend their life in the face of progressive disability. Therefore, sensitive and appropriately timed discussions with patients and their families are essential to try and clarify patient wishes in advance.
Advance Care Planning in Neuromuscular Diseases
Optimal timing for end of life discussions in neuromuscular conditions such as ALS is described in the international literature [47]. Several triggers for the potential introduction of such discussions with patients and their families have been suggested (see Box11.4).
With the recent evolution of advance care planning and the increasingly earlier involvement of palliative care teams in the care of patients with NMDs, such discussions may occur earlier and more frequently. Discussions may lead to the development of an advance statement, an advance decision to refuse treatment (ADRT), or the appointment of a person with Lasting Power of Attorney for health and welfare (see Chap.13).
In patients with neuromuscular conditions, advance care planning may play an important role in directing symptom management, especially in the last days and hours of life, and prevent life-prolonging treatments being instigated or continued contrary to their actual preferences. Of importance, at the time an advance care plan is prepared, the patient must be competent, able to process relevant information, and display an appropriate appreciation of the potential consequences of any decisions made [47].
Box 11.3: Indicators of Disease Progression in Neuromuscular Conditions
Decline in respiratory function
Swallowing problems
Recurring lower respiratory tract infections
First episode of aspiration pneumonia
Marked decline in physical status
Cognitive impairment
Weight loss
Significant complex symptoms
Recurring hospital admissions
Adapted from End of life care in long term neurological conditions: a framework for implementation
Box 11.4: Triggers for Initiating End of Life Discussions
1.
The patient opens the discussion for end of life information and/or interventions
2.
Severe psychological and/or social or spiritual distress or suffering
3.
Pain requiring high dosages of analgesic medications
4.
Dysphagia requiring feeding tube
6.
Loss of function in two body regions (regions include bulbar, arms, and legs)
Adapted from Bede et al. [47]
Discontinuation of Non-invasive Ventilation in Neuromuscular Disease
Early discussions with patients and relatives around end of life issues in the terminal phase of NMDs, such as the discontinuation of non-invasive ventilation and the use of documented advance statements are essential for patient-centered decision making [48]. The ability to maintain control over decisions regarding ventilation and in particular its discontinuation is fundamental to patients deliberating over whether to commence ventilation [49].
As neuromuscular disease progresses, patients established on NIV may find that they become increasingly dependent on the ventilator to relieve symptoms of breathlessness, sometimes requiring it up to 24 h/day. During the terminal phase, ventilated patients may find ongoing treatment brings little hope of recovery, but instead prolongs the dying process. It may be appropriate to consider or offer alternative measures to relieve breathlessness (both non-pharmacological and pharmacological) in order to allow patients periods of freedom from the ventilator.
However, the patient may ask for ventilation to be discontinued altogether, with adequate symptom control to minimize any associated distress [46]. In the event that a competent patient who is deemed to have mental capacity and is not suffering from a depressive disorder or a patient with a valid and applicable ADRT requests discontinuation of non-invasive ventilation, then such wishes should and must be respected. Continuation of ventilation, against a patient’s will, would be both ethically and legally indefensible. Elective withdrawal of ventilator support can cause distress and anxiety to all involved and requires sensitive and thoughtful discussion, with patient, relatives, and healthcare professionals [46].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


