24
Research workers
The exposures encountered by research workers are as numerous and varied as the research they do. Thus the term ‘research worker’ is a broad one and encompasses a very wide range of individuals with an unusually wide range of potentially hazardous exposures. Moreover, at least in economically developed countries, the total population of research workers is large. Many researchers will be working in universities or other places of higher education – in some cities these are major employers with many thousands of research staff. Others will be employed in the research departments of the commercial sector, often in the pharmaceutical or biotechnology fields; again these workforces are often large although there is an increasing trend, particularly in the biotechnology sector, towards small companies employing just a few research staff. The term ‘research worker’ might also be applied to those who work in developmental or quality control laboratories within industry.
In this context, any meaningful clinical assessment of a potentially ‘occupational’ lung disease will require close attention to particular exposures relating both to materials that are used in primary research and those that represent finished products. In many cases such exposures will be obvious; these include, for example, research work with laboratory animals, the use of chemicals widely recognized to cause respiratory irritation and the wearing of latex gloves. Other exposures will be more obscure and will require careful questioning and/or consultation of reference information obtainable either through an employer or directly from the producers or suppliers of chemical agents.
Research workers are, in general, highly educated and highly motivated. They will have a better understanding of their work than will their clinician; at the same time they may have a mildly cavalier attitude to the hazards associated with their work, especially if they have been involved in it for many years. Many will be reluctant, at least initially, to consider an occupational etiology for their disease and more reluctant still to consider leaving or changing their work; this poses special difficulties for those who have developed an occupational hypersensitivity. On the other hand their levels of education – and the relatively liberal environments where they work – mean that career options are often wider than for those who work in industry. Most institutes of higher education and most large companies involved heavily in research will have a well organized system of occupational healthcare including regular health surveillance. Thus their employees are, in this respect, relatively well supported. Furthermore, most research settings are carefully regulated and most research processes are on a fairly limited scale. For these reasons, researchenvironment exposures – with some exceptions, notably those relating to laboratory animals – tend to be considerably less intense than those encountered on the shop floor.
24.2 Respiratory hazards and diseases
The most important occupational respiratory diseases in this workforce are those characterized by (variable) airflow limitation (Figure 24.1). Asthma and rhinitis may be induced through sensitization to a workplace agent or, less commonly, by exposure to a toxic dose of a respiratory irritant. Other respiratory diseases arising from research work are far less common. Acute exposures to high doses of respiratory irritants (‘inhalation accidents’) can give rise to toxic damage to the upper and lower airways and even to the gas-exchanging parts of the lung. Irritant exposures of sufficient intensity to cause severe disease appear, fortunately, to be rare in the laboratory. Various forms of interstitial lung disease including hypersensitivity pneumonitis have been attributed to chemical and biological exposures, but again rarely in research workers. More rarely still, in this context, exposures in the laboratory have been considered as contributory to some cases of lung cancer.
Figure 24.1 Overview of occupational respiratory disease in a research setting; RADS* = reactive airways dysfunction syndrome; OB* = obliterative bronchiolitis; COP = (cryptogenic) organizing pneumonia
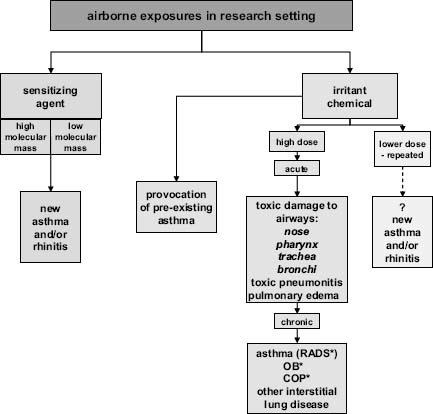
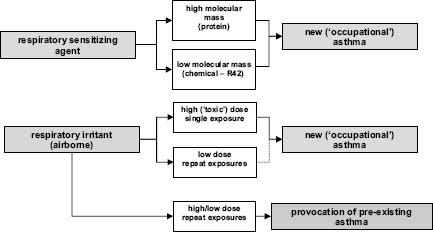
In each case of occupational respiratory disease in a research worker it is helpful to establish precisely the causative agent; and where appropriate to distinguish disease that has arisen de novo as a result of an exposure at work from pre-existing disease that has been provoked by one or more such exposures. Most commonly the need for this distinction arises in cases of work-related asthma. In the strict sense, ‘occupational asthma’ is that which has been induced by a sensitizing or irritant agent in the workplace, while ‘workexacerbated asthma’ is that from another (nonoccupational) cause that is provoked by one or more exposures at work (Figure 24.2). Such a distinction may not be easy but has important diagnostic, management, prognostic, employment and legal implications.
Figure 24.2 Asthma and occupational exposures at work. Note that those with pre-existing asthma can also become sensitized to a workplace agent and develop worsened asthma as a consequence. ‘R42’ is a chemical hazard identification for a recognised respiratory sensitising agent
Most of the common occupational respiratory diseases in research workers are of short – or relatively short – latency. Thus the adverse outcomes of inhalation accidents are generally immediate, with even the longer term sequelae becoming apparent within a few months. The risks of respiratory sensitization are highest within the first few years of first exposure. It is generally easier to establish (or otherwise) an occupational etiology for a disease that is of relatively brief latency. On the other hand they tend to give rise to important employment and related consequences that may be more prominent than for diseases, such as many pneumoconioses, whose onset is only apparent many decades later.
24.3 Respiratory sensitization: asthma and rhinitis
Asthma and rhinitis may readily be induced as immunological reactions to an airborne sensitizing agent in the research workplace. There are many such agents, generally categorized as of high or low molecular mass. The former group is composed largely of proteins, the latter of reactive chemicals. Some groups of research workers are exposed to high concentrations of well recognized respiratory sensitizing agents in the course of their work; examples are given in Table 24.1. The most common are high-molecularmass proteins, particularly but not exclusively those found in the excreta and/or secreta of laboratory animals. Less commonly, researchers may develop respiratory sensitization to an inhaled drug or other reactive chemical. The list in Table 24.1 is by no means exhaustive and any case of asthma or rhinitis arising in a laboratory worker should prompt a search for an occupational etilogy – especially where there is exposure to an airborne protein, any one of which is probably capable of inducing asthma.
Table 24.1 Respiratory sensitizing agents – and the relative frequency with which they cause disease – encountered by research workers
| Agent (examples) | Relative frequency of published cases |
| Laboratory animal proteins | |
| Mice | +++ |
| Rats | +++ |
| Guinea pigs | +++ |
| Hamsters | ++ |
| Ferrets | + |
| Dogs | ++ |
| Cats | + |
| Primates | + |
| Locusts, grasshoppers | ++ |
| Drosophila spp. (fruit fly) | ++ |
| Mealworms | + |
| Butterflies, moths | + |
| Cockroaches | + |
| Serum albumen (bovine, mouse, etc.) | + |
| Animal feedstuff (corncob, etc.) | + |
| Pollen (grass, oil seed rape, sunflower, etc.) | + |
| Latex | ++ |
| Molds (Aspergillus spp., Dictyostelium, etc.) | + |
| Enzymes (papain, pancreatin, xylanase, bromelin, etc.) | + |
| Egg white | + |
| Drugs (piperazine, penicillins, morphine, cimetidine, etc.) | + |
| Cleaning agents (glutaraldehyde, benzyl ammonium chloride, etc.) | + |
| Other chemicals (ninhydrin, iso-nonayl oxybenzene sulfonate, etc.) | + |
Laboratory animal allergy in those who carry out in-vivo animal research is common, with a prevalence of around 15% and an annual incidence of about 5% among those with regular exposure. The risks are higher in those with highest exposure and in those with other atopic disease such as hayfever or cat allergy. Most cases now arise from contact with mice, reflecting the increased use of this species in medical and pharmaceutical research. Other commonly implicated species are rats, guinea pigs and larger mammals such as dogs (Table 24.1). Sensitization to primates appears to be rare. The major allergens in small mammals are found in their urine and are easily transmitted to the pelt and to bedding material and thus, when dried, become airborne. Direct handling of whole animals, anesthetized or otherwise, and changing bedding (cage cleaning) are common causes of respiratory exposure. Many animal research workers will have only intermittent exposure relating to the timing of their experiments; those tasked with animal husbandry are likely to have higher and more consistent exposures.
Chemicals that are recognized to induce respiratory sensitization should carry a specific label, generally to be found on the relevant safety data sheet. Currently their label is ‘R42’ (‘may cause sensitization by inhalation’); with the soon to be enacted ‘Globally Harmonized System’ for chemical classification this will become ‘H334’.
Lists – albeit incomplete – of biological and chemical agents that have been reported to cause occupational respiratory sensitization are available in print (see Further Reading) or via the web (www.asmanet.com, www.asthme.csst.qc.ca, www.eaaci.net). Additionally there is increasing interest in the structural characteristics that distinguish (inorganic) chemicals that are capable of inducing respiratory sensitization. Quantitative structure-activity relationship analysis of such chemical is becoming increasingly sophisticated and appears to have near-perfect negative predictive value. Access to analysis of this sort and appropriate interpretation is freely available on the web and may prove helpful when considering the etiological role of an unfamiliar chemical (http://homepages.ed.ac.uk/jjarvis/research/hazassess/hazassess.xhtml).
24.4 Making a diagnosis of respiratory sensitization
The clinical manifestations of laboratory animal allergy are well recognized and are instructive. They probably apply to most other causes of respiratory sensitization among research workers:
- Most cases arise within two years of first exposure, a reflection of the responsible immune process and of innate individual susceptibility. It is unusual for a laboratory animal researcher to develop disease after many years of similar work. Beware, however, the researcher who has been employed for many years but has recently started work with a different species or process, and the researcher who has elected previously to deny the presence of work-related symptoms.
- For much the same reasons, laboratory animal allergy does not generally manifest within the first few months of exposure. This period of latency is an important clinical clue to the distinction of ‘occupational’ asthma from the exacerbation of asthma due to another cause.
- Symptoms of asthma due to animal sensitization are universally accompanied by nasal and eye symptoms similar to those that characterize hayfever. Thus the absence of eye and nose symptoms at work makes a diagnosis of laboratory animal asthma improbable.
- Both lower and upper respiratory symptoms are related to exposures at work in as much as they are provoked by being at work and are, at least in early cases, relieved when away from work. Immediate (‘early’) symptoms may be accompanied by those that occur later (‘delayed’), perhaps while at home after work. This can give rise to some diagnostic confusion. In more chronic cases there may be relatively little – or no – relief when away from work, a reflection of ongoing bronchial and nasal hyper-reactivity.
- Similarly, hyper-reactivity as a result of immunological sensitization may also give rise to symptoms on contact with nonspecific and nonoccupational irritant exposures such as perfumes or cold air; again this may confuse the clinical picture.
- Laboratory animal asthma – and most cases of laboratory animal rhinoconjunctivitis – is almost always accompanied by evidence of specific immunological sensitization. This is characterized by the production of specific IgE antibodies and is detectable by skin prick testing with appropriate allergens or by measurement of specific IgE antibodies in serum. Suitable reagants for many animal species are available commercially but in more unsusual cases it may be necessary to communicate directly with an experienced laboratory. For small mammals, testing with urinary antigens is more sensitive than testing with epithelial extracts. Skin prick testing should include the use of positive and negative control solutions; the latter is especially important to avoid false positive findings.
- Once sensitized, patients with laboratory animal allergen may be exquisitely sensitive to even very small concentrations of airborne allergen. This is reflective of the hypersensitive immunology that gives rise to the disease; it can make management of an individual case very difficult.
The diagnostic process in research workers suspected of laboratory animal allergy reflects the manifestations and processes listed above. History taking requires careful attention to the nature and duration of appropriate exposures. It is helpful especially to enquire:
- What exactly is their occupational contact with animals in terms of direct handling of live, anesthetized or dead animals? Are they handling only harvested tissues? For how long and how frequently do they have such contact? Does their work include cleaning cages or the handling of bedding or foodstuffs? In occasional cases sensitization arises not from animal proteins but from biological material used as feed.
- How often are they in the animal facility without direct animal contact? Is their animal work only within the animal house or do they also carry out experiments in other laboratories?
- How close and how often are they working to and with others who handle animals? Similarly, is this only within the animal house or does it occur in other settings?
- Do they wear latex gloves when working with animals? Where powdered gloves are (or have been) used, then these may be responsible for immune sensitization (latex allergy) rather than the animal species which they are used to handle.
- Do they wear protective respiratory equipment when working with animals? How often do they wear it – and how often is it serviced? Are simple half-face masks re-used and in any case do they fit properly? Some animal research facilities require the use of powered full-face ventilators; if used routinely and properly, these are extremely effective in reducing exposures to airborne allergens.
- What has been their previous experience with laboratory animal work – including as an undergraduate? Do they – or have they in the past – kept similar animals as pets at home? Pet rats, for example, are remarkably adept at inducing sensitization in their domestic owners.
While it is possible to measure air levels of, and personal exposures to, animal allergens within the laboratory the methodology is complicated, poorly standardized and seldom if ever useful in clinical practice.
In the same way as above, a careful account of the character, latency and timing of symptoms is crucial. Here, helpful information includes:
- A history enquiring into previous respiratory allergy – even if quiescent at the time of starting work. It is of course perfectly possible to acquire an occupational asthma or rhinitis on the back of a prior history of such allergic disease; disentangling the two can be difficult.
- The timing of first symptoms at work. Those that begin very shortly after starting a new job are likely to reflect the provocation of pre-existing disease or asymptomatic bronchial hyper-reactivity. Those that start several months later are more likely to reflect immune sensitization.
- The use of asthma or nasal treatments and an assessment of the degree of control of any pre-existing condition.
- The co-existence of work-related nasal and lower respiratory symptoms and their relative timing of onset. Classically in laboratory animal allergy rhinitis is reported before the onset of asthma symptoms.
- The current temporal relationship between symptoms and work. Recall that any improvement away from work may be less obvious if symptoms have been present for a long time.
- The current temporal relationship between symptoms and specific tasks at work. Advantage can be taken of the often very variable daily schedule of many research workers. Note that patients with bronchial hyper-reactivity may relate their symptoms to irritant exposures at work. Most respiratory irritants do not induce specific sensitization and reactions to them may reflect an immunologically induced bronchial hyper-reactivity to another agent.
It is unwise to make a diagnosis of animal allergy on the basis of a history alone; such a practice will inevitably give rise to false positive diagnoses. In the presence of a characteristic history from an animal research worker with appropriate exposure, the next step is the establishment or otherwise of specific sensitization using, as above, either skin prick testing or measurement of serum specific IgE antibodies – or preferably both. The absence of an identifiable IgE response – assuming this has been done rigorously – should prompt serious consideration of an alternative diagnosis.
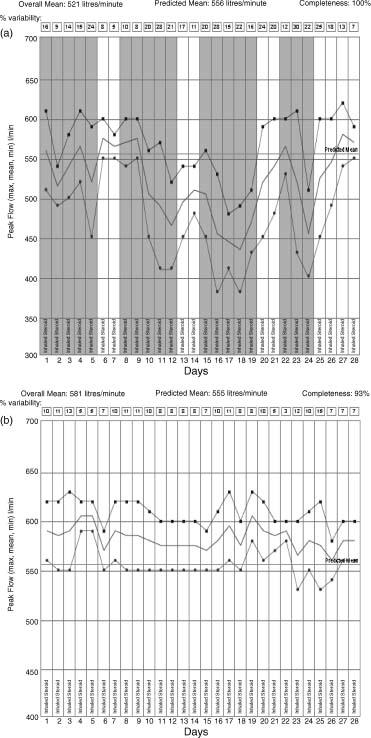
Conversely the presence of IgE sensitization alone is insufficient to make a diagnosis of laboratory animal asthma since there clearly exists a state of ‘asymptomatic sensitization’. Even with an appropriate history and with evidence of IgE sensitization, most clinicians will search for further evidence of an occupational etiology. This is most easily done through measurements of peak expiratory flow serially at home and work; the sensitivity and specificity of this diagnostic approach is considerably enhanced (to about 75 and 90% respectively) if measurements are made more than four times a day for a period of at least a month. By plotting daily mean, maximum and minimum values and comparing these between periods at home and at work it is generally possible to establish – or disprove a functional relationship with work (Figure 24.3a).
Figure 24.3 (a) Serial peak flow record in research worker with prior sensitization to rat urinary proteins. Days at work are depicted by shaded columns, those away from work by unshaded columns. On each day only the mean (solid line), maximum (squares) and minimum (circles) values of six daily readings are shown. There is clear evidence of a fall in peak flow and increased diurnal variability (see boxes) when the patient is at work. Note: during this record the patient had no direct contact with rats and nor did he enter a room where rats were being held. The changes in peak flow and his accompanying symptoms of asthma were caused by very low exposures encountered in the corridors adjacent to rat-containment rooms (see also Figure 24.4). Only by complete avoidance of the workplace was he able to abolish his symptoms – and record a flat peak flow record (b)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


