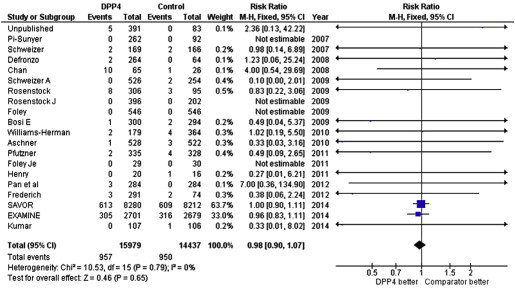
We would like to thank Buisson et al for discovering the error in our previous meta-analysis and commend them for their diligence. We have reviewed the raw numbers of major adverse cardiovascular events (MACE) from our previous meta-analysis and confirmed that the MACE we recorded from the report by Chan et al were erroneous. In retrospect, the 2 abstracters recording this information both inadvertently used the events listed in Table 5 of the report by Chan et al, which depicted projected events per 100 patient-years of therapy (10 and 12 for sitagliptin and control groups, respectively), rather than actual events as listed in Table 4 of the report by Chan et al (11 and 1 for sitagliptin and control groups, respectively). After correcting this numerical error, the point estimate for the pooled analysis showed that DPP4 inhibitors no longer had a statistically significant effect on MACE, as pointed out by Buisson et al.
Since the time of our initial analysis, 2 large prospective randomized controlled trials of DPP4 inhibitors have been published. Therefore, we have taken the opportunity to update our meta-analysis by adding in these new data regarding MACE from the SAVOR-TIMI 53 and EXAMINE studies, which evaluated saxagliptin and alogliptin, respectively. The updated pooled analysis ( Figure 1 ) shows that DPP4 inhibitors had a neutral effect on MACE (relative risk [RR] 0.98 [0.90 to 1.07], p = 0.65), cardiovascular death (RR 0.96 [0.83 to 1.11], p = 0.57), and myocardial infarction (RR 0.98 [0.86 to 1.11], p = 0.70). The addition of these new randomized trial results markedly improves the power of our previous meta-analysis. Thus, the cumulative randomized trial data suggest that DPP4 inhibitor therapy neither increases nor decreases the risk of adverse cardiovascular events ( Figure 2 ).