Fig. 16.1
The inflammatory phase of cardiac repair. Release of damage-associated molecular patterns (DAMPs) by dying cardiomyocytes and matrix fragments activates innate immune pathways leading to recruitment of leukocytes in the healing infarct. Mast cells, neutrophils and mononuclear cells, but also endothelial cells, fibroblasts and cardiomyocytes contribute to the pro-inflammatory environment
16.2.2 Role of Complement Activation in Post-infarction Inflammation
The complement system is a first line of defense with a central role to the innate immune response. Involvement of the complement system in MI was first demonstrated in a rat MI model by Hill and Ward [7]. Since then, numerous studies documented that activation of complement is an early event in postinfarction inflammation and healing [8]. In the infarcted myocardium, subcellular substances released by dying cardiomyocytes (such as cardiolipin) can activate complement. On the basis of extensive experimental evidence, inhibition of the complement system seems to be a promising therapeutic target to reduce myocardial injury and limit the infarct size. Consumptive depletion of complement [9], antibody-induced inhibition of individual complement components (e.g. C5), or infusion of modified native complement components that block complement activation exhibited protective actions in experimental animal models of AMI [10, 11]. The effectiveness of such approaches in animal models generated considerable interest leading to clinical trials testing the effectiveness of C5 inhibition in patients with MI. Most clinical studies have focused on pexelizumab, a humanized single chain variable fragment (scFv) to complement C5. Although some favorable results of pexelizumab were reported as adjunctive therapy to reduce reperfusion injury in coronary artery bypass surgery [12], most of the clinical data, showed no benefit in patients with AMI [13].
16.2.3 The Role of ROS Generation
Reactivate Oxigen species (ROS) are atoms or molecules with unpaired electrons in their outer orbit; they are highly reactive entities and can participate in a variety of biochemical reactions [14]. ROS react directly with cellular lipids, proteins and DNA causing cell injury and death, and are critically involved in the oxidative burst reaction, which is essential for phagocyte function. The normal heart possesses substantial ability to counterbalance the generation of ROS through inhibitory enzymatic pathways and through activation of intracellular antioxidants. The antioxidant defenses, however, are overwhelmed following infarction, resulting in net generation of free radicals that may cause myocardial contractile dysfunction and structural damage [15]. Generation of ROS can activate the immune cells in the infarcted heart and trigger cytokine and chemokine release partially through activation of NF-κB system [16]. Overactive ROS-mediated signaling has the potential for detrimental actions in the infarcted heart mediated through matrix degradation and by inducing cardiomyocyte apoptosis.
Free radical scavengers have been widely used to study the role of ROS in the pathophysiology of MI; their effects generated interest in possible clinical applications. Jolly et al. [17] demonstrated protective effects of combining the antioxidant enzymes superoxide dismutase (SOD) and catalase in reduction of infarct size in dogs undergoing coronary occlusion and reperfusion. Recently, transgenic mice with specific overexpression of extracellular SOD (ecSOD) in cardiomyocytes exhibited significant protection from post-ischemic/reperfusion injury [18]. In addition, mice overexpressing manganese SOD (MnSOD) demonstrated a significant decrease in infarct size in Langendorff-perfused hearts undergoing coronary artery ligation [19]. However, not all experimental evidence suggests a deleterious effect of ROS in the infarcted heart [20]. The timing of administration, the dosage of the antioxidant, and the pleiotropic effects of ROS may be responsible for contradictory findings in experimental studies. Thus, it is not surprising that studies using antioxidant therapy in myocardial infarction have produced discouraging results in the clinical arena. A small clinical study using recombinant human SOD in patients with AMI undergoing balloon angioplasty [21] demonstrated no significant improvement in left ventricular function.
16.2.4 TLR Signaling in the Post-infarction Inflammatory Reaction
DAMPs exert their pro-inflammatory actions by activating members of the TLR family; binding of danger signals to the respective TLRs induces activation of several kinases and of the NF-κB cascade. Of the 13 known mammalian TLRs, TLR-2, -3 and -4 has been identified to play an important role in mediating the inflammatory response in the infarcted heart [22–24]. Deletion of TLR-4 [25] or disruption of TLR-2 signaling [23] by specific antibodies have decreased infarct size and suppressed inflammation following MI, identifying TLR-2 and -4 as key components of the innate immune response in the heart. Thus, TLR-dependent activation of inflammatory cells plays an important role in the innate immune response following MI.
16.2.5 NF-κB Activation in Myocardial Infarction
TLR-mediated, complement-activated and ROS-induced pathways converge on the activation of the transcription factor NF-κB, an important signaling component for early inflammatory activation that triggers cytokine, chemokine and adhesion molecule expression in the ischemic myocardium. Activation of the NF-κB system has been demonstrated in models of experimental myocardial ischemia and reperfusion. Although some studies have suggested injurious pro-inflammatory actions of NF-κB in the infarcted myocardium, other investigations indicated that the NF-κB pathway may also mediate cytoprotective responses in the ischemic heart [26]. Activation of the NF-κB signaling cascade in multiple parallel processes involving various cell types, critical for infarct healing, complicates understanding of its role in myocardial infarction. In vivo studies examining the role of cell-specific NF-κB activation at different stages of the reparative response would greatly contribute to our understanding of cardiac injury and repair.
16.2.6 Inflammatory Signals and Cellular Events During the Inflammatory Phase of Infarct Healing: Chemokine Signaling in the Infarcted Heart
Induction of chemokines is a prominent feature of the post-infarction inflammatory response [27–29]. The chemokines are small polypeptides with molecular weights in the range of 8–14 kDa that play an important role in leukocyte trafficking. From a structural perspective, chemokines are divided into subfamilies on the basis of the number and sequential relationship of their conserved cysteine residues (CXC, CC, XC and CX3C chemokines) [3]. CXC chemokines that contain the ELR (elastin-like recombinamer) motif, such as IL-8/CXCL8, mediate neutrophil migration, whereas CC chemokines, such as Monocyte Chemoattractant Protein (MCP)-1/CCL2 recruit mononuclear cells. The effects of chemokines extend beyond actions on cells of hematopoietic origin. For example, the CXC chemokine Stromal Derived Factor (SDF)-1 is implicated in angiogenesis and fibrous tissue deposition [30]. On the other hand, another ELR-negative CXC chemokine, Interferon-inducible Protein (IP)-10/CXCL10 exerts direct inhibitory actions on fibroblast migration, serving as an antifibrotic agent [31], and may also act as an angiostatic mediator [32]. Using a mouse model of reperfused infarction, our laboratory demonstrated that endogenous IP-10/CXCL10 is an essential inhibitory signal that regulates the cellular composition of the healing infarct, preventing uncontrolled fibrosis and attenuating adverse REM through direct actions on fibroblast migration and function [33].
Monocyte Chemoattractant Protein (MCP)-1/CCL2 is the best-studied CC chemokine. In addition to its critical role in mononuclear cell recruitment, MCP-1 has been suggested to exert important actions on non-hematopoietic cells, inducing angiogenic and arteriogenic effects [34] and modulating fibroblast phenotype and activity by increasing collagen expression and by regulating matrix metalloproteinase synthesis [35]. Using both antibody neutralization studies and genetic disruption models, we have documented a crucial role of MCP-1 on macrophage recruitment and activation, cytokine synthesis and myofibroblast accumulation in healing infarcts [36]. MCP-1 −/− mice demonstrated decreased and delayed macrophage infiltration accompanied by impaired phagocytosis and delayed replacement of injured cardiomyocytes by granulation tissue in the healing infarct.
16.2.7 Expression and Role of the Cytokines in Myocardial Infarction
Activation of cytokine cascades is a prominent characteristic of the post-infarction inflammatory reaction. Complement activation, TLR signaling, free radical generation and NF-κB activation are capable of stimulating cytokine expression in both resident and blood-derived cells, resulting in marked cytokine upregulation in the infarcted area. Cytokines are highly pleiotropic and are capable of modulating phenotype and function of all cell types involved in cardiac repair. Their multifunctional and context-dependent properties have hampered understanding of their role in the infarcted myocardium.
As the prototypical pro-inflammatory cytokine, interleukin (IL)-1 mediates synthesis of other cytokines, chemokines, growth factors, and adhesion molecules in the infarct and stimulates leukocyte recruitment [37]. Marked IL-1β upregulation has been reported in experimental models of MI and in patients suffering an AMI [38]. Activation of the inflammasome, the molecular platform involved in IL-1 activation, is noted in both cardiomyocytes and non-cardiomyocytes in the infarcted heart [39, 40]. IL-1 is a central mediator in the post-infarction inflammatory response; disruption of IL-1 signaling markedly reduces infiltration of the infarcted myocardium with leukocytes and attenuates MMP expression decreasing dilative REM [37]. IL-1 also critically regulates fibroblast phenotype in the infarct, delaying conversion of fibroblasts into myofibroblasts and promoting a matrix-degrading phenotype [41]. Because of its central role in mediating post-infarction INFL, matrix degradation and dilative REM, the IL-1 system may be a promising therapeutic target in patients with AMI [42].
The pro-inflammatory cytokine Tumor Necrosis Factor-α (TNF-α) is released early in the infarcted myocardium [43], and (much like IL-1) may stimulate expression of other inflammatory mediators by leukocytes and endothelial cells. TNF-α deficient mice undergoing MI protocols had reduced expression of chemokines and adhesion molecules suggesting an important role for TNF-α in mediating the post-infarction inflammatory response [44]. However, as a highly pleiotropic mediator, TNF-α does not simply serve as a trigger of a cytokine cascade, but may also modulate cell survival pathways. Experimental studies exploring effects of TNF signaling on cardiomyocyte survival have produced contradictory results. Kurrelmeyer et al. [45] demonstrated that TNFR1/TNFR2 double receptor knockout mice undergoing left coronary artery ligation had significantly increased infarct size exhibiting accentuated cardiomyocyte apoptosis when compared with wild-type controls. In contrast, Sugano et al. [46] reported that reduction of bioactive TNF-α through local delivery of sTNFR1 inhibited cardiomyocyte apoptosis in a rat model of ischemia/reperfusion. Attempts to implement anti-TNF approaches in patients with AMI have been disappointing, possibly reflecting the pleiotropic actions of the cytokine that may exert both injurious effects (through accentuation of inflammation) and protective pro-survival actions on cardiomyocytes.
16.2.8 The Cellular Immune Response in the Infarcted Myocardium
16.2.8.1 The Neutrophils
Early recruitment of abundant neutrophils is a hallmark of the post-infarction inflammatory reaction. Neutrophils release oxidants and proteases, secrete mediators that may amplify inflammatory cell recruitment, and phagocytose dead cells and debris. Early experimental studies suggested that neutrophil depletion in ischemia-reperfusion models markedly reduced infarct size [47] suggesting a role for neutrophils in extension of ischemic injury. However, more recent studies using genetic models associated with marked reduction in neutrophil infiltration in the infarct did not confirm these observations suggesting that neutrophil-mediated injury may not increase the size of the infarct [48].
Neutrophil transmigration in the infarcted myocardium requires specific interactions between the leukocytes and the endothelium, mediated through activation of a multistep adhesive cascade (Fig. 16.1). Each step of the cascade requires either upregulation, or activation, of distinct sets of adhesion molecules. First, leukocytes are captured by endothelial cells or roll on the endothelial surface via interactions that involve members of the selectin family. The selectin family includes L-, P- and E-selectin. L-selectin (CD62L) is expressed in neutrophils, whereas P-selectin (GMP-140, CD62P) and E-selectin (CD62E) are expressed in the endothelial surface. Although extensive evidence suggests the role of the selectins in supporting leukocyte margination under shear stress, the effects of selectin-related interventions in experimental models of myocardial ischemia have been inconsistent [48].
As leukocytes roll on the endothelium through selectin-mediated actions, they “sense” chemokines immobilized on the endothelial surface and engage into a firm adhesive interaction with endothelial cells through activation of the integrins [49]. The integrins are a family of heterodimeric membrane glycoproteins that consist of an α and a β subunit. Activated neutrophil β2 (CD18) integrins interact with endothelial Intercellular Adhesion Molecular (ICAM)-1 resulting in firm adhesion of leukocytes into the vascular endothelial layer. After firm adhesion, transmigration of activated neutrophils follows. The transmigration step is dependent on adhesion molecules, such as ICAM-1, Vascular endothelial (VE)-cadherin and members of the Junctional Adhesive Molecular (JAM) family. Because of the critical role of the integrins in neutrophil adhesion and transmigration, integrin-targeting strategies have been utilized to mitigate post-reperfusion inflammation in various experimental models. In animal models of experimental MI, inhibition of CD11/CD18 integrin resulted in significant reduction of infarct size [50]. However, despite the promising findings of the experimental studies, in small clinical trials leukocyte integrin inhibition was not effective in reducing infarct size and acute injury [51].
16.2.8.2 Monocytes and Macrophages
Monocytes and macrophages are key cellular effectors in the post-infarction inflammatory and reparative response [52]. Distinct monocyte subpopulations are sequentially recruited in the infarcted myocardium [53]. The CC chemokine CCL2/MCP-1 plays an important role in chemotactic attraction of pro-inflammatory monocytes that express the chemokine receptor CCR2 and have prominent phagocytic properties [36]. Other mediators including complement, Transforming growth factor (TGF)-β, free radicals and other CC chemokines may also play a role in regulating monocyte infiltration; their role in recruitment of specific monocyte subsets remains unknown. The recruited monocytes are predominantly bone marrow-derived; however, in mouse models extramedullary sources (such as the spleenic monocyte reservoir) may also mobilize monocyte subpopulations [54, 55]. Recruited monocytes undergo phenotypic changes and may differentiate into macrophages (Fig. 16.2) in response to microenvironmental factors, such as cytokines and growth factors. The maturation of monocytes into mature macrophages is a complex and poorly understood process, that involves growth factors such as Macrophage-Colony Stimulating Factor (M-CSF) and Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) [56]. In addition to the clearance of the infarct from dead cells and matrix debris, macrophages have several additional functions including: (a) the production of a wide range of growth factors and cytokines that stimulate fibroblast and endothelial cell proliferation and may regulate the healing response and angiogenesis and (b) remodeling of the extracellular matrix network through the production of matrix metalloproteinases and their inhibitors.
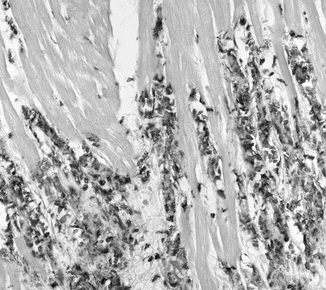

Fig. 16.2
Immunohistochemical staining with the macrophage-specific antibody PM-2K identifies an abundant macrophage population in canine infarcts after 1 h of coronary occlusion and 72 h of reperfusion
16.2.8.3 The Mast Cells: Versatile Cells with a Potential Role in Infarct Healing
Mast cells are multifunctional cells that contain a wide range of mediators, including cytokines, growth factors, tryptase, chymase and histamine. Due to their strategic location, mast cells are likely to play an important role in initiating the inflammatory response through the release of pro-inflammatory mediators, capable of triggering the cytokine cascade. Mast cells release histamine and TNF-α in the early stages of the ischemic response and may play an important role in initiating the cytokine cascade [43]. As the infarct heals, induction of Stem Cell Factor (SCF) attracts mast cell progenitors, leading to a marked increase in mast cell numbers in the infarcted area (Fig. 16.3) [57]. Mast cell-derived mediators, have potent pro-inflammatory, angiogenic and fibrogenic actions. Although other cell types can produce growth factors and cytokines, histamine and the proteases tryptase and chymase are uniquely secreted by mast cells and may play an important role in cardiac repair [58].
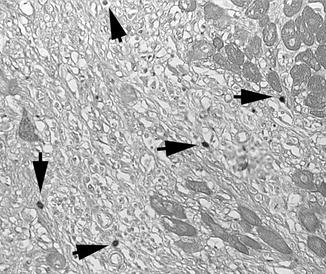

Fig. 16.3
Toludine blue staining identifies mast cells accumulating in the infarcted canine heart (arrows)
16.3 The Proliferative Phase of Healing
16.3.1 Resolution of the Post-infarction Inflammatory Response
During the inflammatory phase of infarct healing, infiltration with activated neutrophils and mononuclear cells plays a crucial role in phagocytotic removal of dead cells and debris. However, effective repair is dependent on timely repression and resolution of the inflammatory reaction and on spatial containment of post-infarction inflammation in the infarcted area. Unrestrained, prolonged or expanded inflammatory activity could have catastrophic consequences in the injured heart, leading to adverse dilative REM and to the development of systolic and/or diastolic dysfunction. Repression of inflammation is an active process that requires timely activation of endogenous “STOP” signals that suppress the inflammatory reaction and trigger reparative cascades. From a cell biological perspective, timely resolution of inflammation following MI requires the coordinated actions of several different cell types including neutrophils, mononuclear cells, endothelial cells and pericytes. Alterations in the extracellular matrix network also participate to the resolution of inflammation after myocardial infarction.
16.3.2 Activation of Inhibitory “STOP” Signals in the Infarcted Heart: Expression of Decoy Receptors to Terminate Inflammation in Myocardial Healing
Cytokines and chemokines play important roles in post-infarction inflammation by interacting with their receptors, thus activating downstream intracellular pathways. However, for several members of the cytokine and chemokine family, receptors have been identified that bind to the corresponding inflammatory mediators with high affinity, but do not transduce a signal, thus serving as a molecular trap to remove the cytokine or chemokine from the local microenvironment. Although induction of decoy receptors to limit INFL is an intriguing mechanism for negative regulation of the inflammatory reaction, the role of such receptors in MI remains largely unknown. D6 is a chemokine decoy receptor that specifically binds and scavenges inflammatory CC-chemokines. Recently, Cochain et al. [59] found that D6 is upregulated in the infarcted myocardium, and that D6 −/− mice have increased chemokine levels in the infarct heart associated with enhanced infiltration with neutrophils and monocytes. As a result of the accentuated inflammatory reaction, D6 null mice were more susceptible to cardiac rupture prone after myocardial infarction. These findings suggest that induction of decoy receptors may protect the infarcted heart from uncontrolled inflammation following infarction.
16.3.3 Activation of Intracellular Pathways That Inhibit the Innate Immune Response
As uncontrolled TLR activation may stimulate a broad spectrum of inflammatory signals, several distinct pathways have evolved to negatively regulate TLR-mediated responses. Interleukin-1 receptor associated kinase (IRAK)-M, is a member of the IRAK family, predominantly expressed in macrophages, that lacks kinase activity but functions as a decoy to limit TLR- and IL-1 mediated inflammatory responses [60]. Our recent experiments demonstrated that IRAK-M is upregulated in macrophages and fibroblasts in the infarcted myocardium. IRAK-M null mice showed accentuated dilative REM with increased infiltration of pro-inflammatory monocytes and overactived MMP activity, suggesting that endogenous IRAK-M may attenuate adverse postinfarction remodeling by suppressing inflammatory activity and by inhibiting fibroblast-mediated matrix degradation in the infarcted heart [61].
16.3.4 The Role of Soluble Inhibitory Mediators in Suppression of the Post-infarction: Inflammatory Response the Role of TGF-Β
TGF-β is a multifunctional cytokine that exerts a wide range of biological effects in regulation of cell proliferation, differentiation, and apoptosis and has profound modulatory actions in the immune response. Because of its effects on both inflammatory and reparative cells, TGF-β is ideally suited as a “master switch” mediator in the transition from inflammation to fibrous tissue deposition in healing infarcts. TGF-β suppresses cytokine and chemokine synthesis by stimulated macrophages and endothelial cells, while promoting myofibroblast transdifferentiation and activation [62], In addition, TGF-β exerts matrix-preserving actions, decreasing expression of proteinases and increasing synthesis of proteinase inhibitors, such as Plasminogen Activator Inhibitor (PAI)-1 and TIMP-1. Unfortunately, the complex biology of TGF-β activation and its multiple cellular actions have hampered our efforts to understand its role in infarct healing.
TGF-β is markedly upregulated in MI and is predominantly localized in the infarct border zone [63]. Early TGF-β inhibition has deleterious consequences on the infarcted heart, presumed due to enhanced pro-inflammatory cytokine synthesis [64]. Thus, timely activation of TGF-β signaling may be important for suppression of inflammatory mediator synthesis following infarction. GDF-15 belongs to the TGF-β superfamily, is induced in the infarcted heart and has been reported to suppress chemokine-triggered integrin activation, thus limiting the inflammatory leukocyte adhesion to the endothelium. GDF-15 null mice undergoing coronary artery ligation protocols exhibited enhanced recruitment of neutrophils into the infarcted myocardium and an increased incidence of cardiac rupture [65], suggesting an important role of this growth factor in negative regulation of the post-infarction inflammatory reaction.
16.3.5 The Potential Role of IL-10
IL-10, a cytokine with potent anti-inflammatory properties, is predominantly expressed by activated Th2 lymphocytes and a subset of macrophages infiltrating the infarct [66]. Its expression shows a late and prolonged time course in the infarcted canine and mouse heart [66]. Monocytes and macrophages are particularly responsive to the anti-inflammatory actions of IL-10. In addition, IL-10 may play a significant role in extracellular matrix remodeling by promoting Tissue Inhibitor of Metalloproteinases (TIMP)-1 synthesis, leading to stabilization of the matrix [67]. Yang et al. demonstrated that IL-10 −/− mice had an enhanced inflammatory response and increased mortality following myocardial infarction [68]. In contrast, our laboratory found comparable survival and post-infarction remodeling in WT and IL-10 null mice undergoing reperfused infarction protocols. When compared with WT animals IL-10 null mice had elevated myocardial MCP-1 and TNF-α expression [69]; suggesting a relatively subtle effect of IL-10 in repression of the post-infarction inflammatory reaction that is not critical for the reparative response.
16.3.6 The Extracellular Matrix as a Regulator of the Post-infarction Inflammatory Reaction
16.3.6.1 Removal of Matrix Fragments Modulates the Inflammatory Response in Healing Myocardium
Extracellular matrix proteins not only provide structural and mechanical support to the tissue, but also modulate cell signaling through interactions with specific surface receptors. During infarct healing, the cardiac extracellular matrix undergoes dynamic changes that drive the inflammatory and reparative response [70]. During the first few hours after MI, matrix fragments are generated in the infarcted area and stimulate the inflammatory mediator synthesis. Evidence from our lab suggested that hyaluronan, a key component of the cardiac extracellular matrix, may undergo degradation in the infarcted area, leading to accumulation of pro-inflammatory low molecular weight hyaluronan fragments in infarcted area. The hyaluronan fragments may activate endothelial cells and macrophages inducing synthesis of cytokines and chemokines. Removal of the low molecular weight hyaluronan fragments from the infarcted area attenuates inflammation in the infarcted heart triggering inhibitory signals through interactions involving CD44 [71].
16.3.6.2 The Role of the Matricellular Proteins
Matricellular proteins are a family of structurally unrelated macromolecules that do not play a structural role, but bind to the matrix and modulate cell-cell and cell-matrix interactions [72]. Temporally and spatially restricted induction and deposition of matricellular proteins is observed in the proliferative phase of infarct healing. Our laboratory has demonstrated that the matricellular protein thrombospondin (TSP)-1, a crucial TGF-β activator with potent angiostatic and anti-inflammatory properties, is strikingly upregulated in the border zone of the infarct [73]. TSP-1 null mice exhibited enhanced and prolonged expression of chemokines in the infarcted heart and showed expansion of the inflammatory infiltrate into the noninfarcted area. These findings suggest an important role for TSP-1 in suppression and containment of the inflammatory reaction following infarction. Therefore, localized induction of TSP-1 in the infarct border zone may form a “barrier” preventing expansion of the inflammatory infiltrate into the noninfarcted area. Other matricellular proteins, such as TSP-2, SPARC/osteonectin, osteopontin (OPN), tenascin-C and periostin are also upregulated in the infarcted heart and modulate the reparative response (Fig. 16.4) [72].
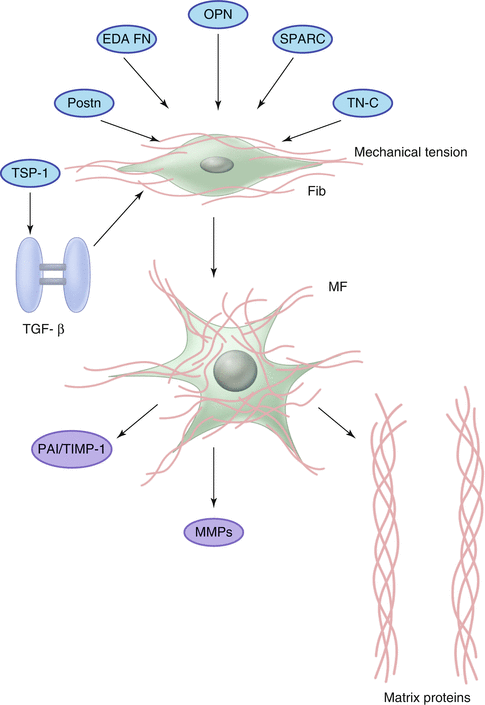

Fig. 16.4
The matricellular proteins osteopontin, SPARC, tenascin-C, periostin and thrombospondin-1 regulate fibroblast phenotype and activity in the healing infarct. Other specialized matrix proteins (such as ED-A fibronectin) also play a critical role in myofibroblast conversion
16.3.7 The Cellular Effectors in Resolution of the Post-infarction Inflammation
16.3.7.1 Macrophage-Mediated Clearance of Apoptotic Neutrophils in Resolution of Post-infarction Inflammation
Clearance of apoptotic cells in the injured tissue may be a crucial cellular mechanism for resolution of inflammation. Neutrophils infiltrating the infarct are programmed to undergo apoptosis, and clearance of apoptotic neutrophils by phagocytes ingesting is associated with active suppression of inflammation and release of large amounts of inhibitory mediators. Apoptotic granulocytes attenuate inflammation in healing myocardium by releasing mediators that inhibit further neutrophil recruitment, or by activating an anti-inflammatory program in macrophages. Macrophages phagocytosing neutrophils release large amounts of IL-10, TGF-β and proresolving lipid mediators [2]. Although the fundamental biology of the inflammatory response suggests that macrophage-mediated clearance of apoptotic infiltrating neutrophils may be crucial in triggering anti-inflammatory and proresolving signals, direct evidence supporting this concept in vivo is lacking. Recently, Wan and co-workers demonstrated that clearance of apoptotic (efferocytosis) in the ischemic myocardium through activation of myeloid-epithelial-reproductive tyrosine kinase (Mertk) in myeloid cells promotes resolution of inflammation protecting from adverse remodeling [74].
16.3.8 Mononuclear Cell Subsets as Negative Regulators of Inflammation Following Myocardial Infarction
16.3.8.1 Inhibitory Monocytes/Macrophages in Resolution of Inflammation
Two main subsets of monocytes have been identified in mice: (1) a subpopulation of Ly6Chi pro-inflammatory monocytes that exhibit high levels of the CC chemokine receptor CCR2 and low level of expression of the fractalkine receptor, CX3CR1 (Ly6Chi CCR2 hi CX3CR1low); (2) a subset of Ly6ClowCCR2low/negCX3CR1hi resident monocytes. Following myocardial infarction, early induction of CCR2 ligands (such as MCP-1) recruits the proinflammatory Ly6Chi cells into the injured site; these cells secrete inflammatory cytokines and phagocytose dead cells and debris [53]. Inhibiting Ly6Chi monocytes infiltration into the infarcted has been suggested as a promising therapeutic strategy to suppress the inflammatory response in healing myocardium [75, 76]. However, attenuation of pro-inflammatory monocyte infiltration may delay phagocytotic removal of dead cardiomyocytes. CX3CR1 (+) monocytes also infiltrate the myocardium and may play a reparative role; however, the mechanisms of their recruitment are poorly understood [77]. In human patients, CD16- monocytes have the same proinflammatory character as murine Ly6Chi cells. Recently, the presence and localization of CD14 + CD16− and CD14 + CD16+ monocyte subsets was studied in human AMI [78]. In the inflammatory phase after AMI, CD14+ cells were identified in the infarct border zone, adjacent to cardiomyocytes, and consisted predominantly of CD14 + CD16− cells. In contrast, during the proliferative phase, the infiltrate of CD14+ cells was localized mainly in the infarct core, and contained about 60 % CD14 + CD16− cells and 40 % CD14 + CD16+ monocytes. Further clinical data have demonstrated that circulating proinflammatory CD14+/CD16- cells exhibit an early peak in patients with ST elevation myocardial infarction and are negatively correlated with recovery of function 6 months after the acute event [79].
In response to the dynamic alteration in cytokine and growth factor expression in the infarct, the infiltrated monocytes undergo phenotypic changes. Upregulation of macrophage-colony stimulating factor induced monocyte to macrophage differentiation [56]. Macrophages can secrete inhibitory mediators to suppress inflammation during resolution of post-infarction healing. On the other hand, as they phagocytose apoptotic cells, macrophages acquire pro-resolving properties secreting anti-inflammatory mediators. In a somewhat simplified classification, macrophages are classified into pro-inflammatory M1 and inhibitory/reparative M2 subsets. In the healing infarct, M1 macrophages are abundant during the first 3 days after AMI, whereas M2 macrophages represented the predominant macrophage subset after 5 days [80].
16.3.9 Regulatory T Cells in Resolution of Inflammation
Subpopulations of T cells with suppressive properties (such as regulatory T cells/Tregs) may also be involved in negative regulation of post-infarction inflammation. Recent evidence suggested that Tregs infiltrate the infarcted heart and may play an important role in suppression of post-infarction inflammation, ameliorating pathologic cardiac remodeling [81, 82]. Chemokine receptor CCR5 null mice exhibited reduced myocardial infiltration with Tregs, associated with increased inflammation after myocardial infarction, indicating that Tregs may play a role in suppression of postinfarction inflammation [81]. Recently, Tang and his coworker [82] demonstrated that in rats undergoing infarction protocols, cell therapy with Tregs improved cardiac function. In addition, infiltration of neutrophils, macrophages and lymphocytes and expression of TNF-α and IL-1β were also significantly decreased in the rats that received Tregs. Take together, those data demonstrate that Treg cells may serve to protect against adverse ventricular remodeling and may contribute to improve cardiac function after myocardial infarction via inhibition of inflammation, or direct protection of cardiomyocytes.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


