Hypertension represents a major health problem, with an appalling annual toll. Despite the plethora of antihypertensive drugs, hypertension remains resistant in a considerable number of patients, thus creating the need for alternative strategies, including interventional approaches. Recently, renal sympathetic denervation (RSD) using a very elegant, state-of-the-art technique (percutaneous, catheter-based radiofrequency ablation) was shown to be beneficial in patients with resistant hypertension. The pathophysiology of kidney function justifies the use of RSD in the treatment of hypertension. Data from older studies have shown that sympathectomy has efficiently lowered blood pressure and prolonged the life expectancy of patients with hypertension, but at considerable cost. RSD is devoid of the adverse effects of sympathectomy because of its localized nature, is minimally invasive, and provides short procedural and recovery times. In conclusion, this review outlines the pathophysiologic background of RSD, describes the past and the present of this interventional approach, and considers several future potential applications.
Resistant hypertension is defined as uncontrolled blood pressure despite the use of optimal doses of 3 antihypertensive agents, of which 1 is a diuretic. Although several factors and conditions can be identified and corrected, a small percentage of patients with hypertension remain uncontrolled. The high prevalence of hypertension in the general population renders this small percentage significant in terms of actual patient numbers. This combined with several limitations in drug therapy (patient adherence, polypharmacy, and adverse drug events) creates the need for other therapeutic options, beyond existing antihypertensive medications, setting the basis for interventional approaches. The present situation is reminiscent of the 1940s and 1950s, when therapeutic options for hypertension were limited, and radical sympathectomy was popular among hypertension experts. The advent of antihypertensive therapy, the benefits of drug treatment in the Veterans Affairs studies, and the significant adverse effects associated with sympathectomy drove the latter to total obscurity. Recently, activation of the carotid baroreceptors using electrical stimuli (the Rheos System; CVRx, Inc., Minneapolis, Minnesota) is under investigation in phase III studies, with promising results so far. Another breakthrough study using selective renal sympathetic denervation (RSD) marks the return of selective sympathectomy as a potential alternative treatment for resistant hypertension. The sympathetic innervation of the kidney is implicated in the pathogenesis of hypertension through effects on renin secretion, sodium and water reabsorption, and renal blood flow (RBF). In this review, we attempt to summarize the role of renal sympathetic nerves in blood pressure regulation and to outline the pathophysiology of RSD. Moreover, we discuss the past and the present of RSD in the treatment of arterial hypertension and explore potential future applications.
Background
The autonomic control of the kidney is predominantly sympathetic; a dense network of postganglionic sympathetic neurons innervates the kidneys. A schematic representation of renal sympathetic innervation is depicted in Figure 1 .
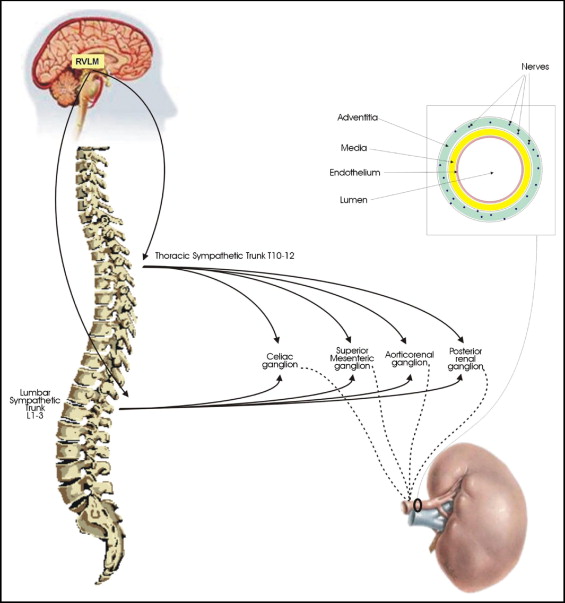
In experimental functional studies, it has been shown that renal sympathetic nerve activation enhances noradrenaline production or spillover, while renal denervation results in a marked decrease of noradrenaline, up to 95%. When renal sympathetic nerves are activated, b 1 adrenergic receptors mediate renin secretion, sodium reabsorption occurs via a 1 adrenoceptors, and renal vessel vasoconstriction takes places via a 1 receptors, resulting in RBF reduction. A frequency-dependent effect of renal sympathetic nerve stimulation has been demonstrated in experimental animals. When the nerve stimulation is weak, renin secretion is observed. If the nerve stimulation is stronger, sodium reabsorption occurs. Finally, the application of much stronger stimulation to renal sympathetic nerves results in RBF reduction. In an attempt to reproduce this frequency-dependent effects in humans, different levels of lower-body negative pressure were applied to gradually activate the sympathetic nervous system (SNS). Increased noradrenaline and plasma renin activity levels were the first to be observed, with reduced sodium excretion found only with increased SNS activity. These effects were demonstrated while RBF and the glomerular filtration rate (GFR) remained unaltered, suggesting that functional effects may appear even in the absence of hemodynamic alterations.
Renal sympathetic nerve endings release noradrenaline directly on granular juxtaglomerular cells and increase the rate of renin release. Moreover, renal sympathetic activation may enhance renin release indirectly by interfering with renal hemodynamics and sodium reabsorption, through renal artery hypotension and decreased influx of sodium chloride into macula densa cells. Two lines of evidence support a direct effect of renal sympathetic nerve activation on renin secretion. First, very low electrical stimulation (0.5 Hz) of renal sympathetic nerves in experimental animals (rats) induces renin secretion, without affecting either sodium reabsorption or RBF. Thus, this weak stimulation may be perceived as a direct effect of sympathetic nerve activation on juxtaglomerular cells, independent of other mechanisms regulating renin secretion. Second, renal denervation abolishes the induction of renin release by conditions that are known to stimulate renin release, such as volume depletion, head-up tilt, and reduced renal perfusion pressure.
Renal sympathetic varicosities release noradrenaline directly on renal epithelial cells and promote the reabsorption of water and sodium from the tubular lumen. In animals, the electrical stimulation of renal sympathetic nerves at low levels that do not affect RBF and the GFR cause a significant reduction (30%–40%) in sodium and water excretion. This effect occurs in most nephron segments but is more pronounced in the proximal tubule and the thick ascending limb of Henle’s loop. It has become apparent from a number of studies that RSD attenuates sodium and water reabsorption, independent of the GFR or RBF, confirming the direct effects of renal innervation on tubular function.
There is sound evidence that renal sympathetic activation results in a significant decrease of RBF. Sympathetic nerve activation results in vascular smooth muscle cell contraction of the resistance vessels and therefore reduces the blood flow through the kidneys. Sympathetic-induced vasoconstriction is more profound in preglomerular than postglomerular microvessels. This imbalance in the vasoconstrictive effects on renal microcirculation represents the main contributor of RBF reduction. The effects of renal denervation on RBF were clearly demonstrated in a study of conscious rabbits, in which RBF was >50% higher in the denervated compared with the innervated kidney 1 week after renal denervation. Human studies with psychological stimuli (nonvertebral IQ testing) that activate the SNS further elucidate the effects of renal sympathetic activation on RBF. It has been shown that RBF was substantially reduced in patients with hypertension by these tests, while it remained unaltered in normotensive subjects. Although the effects of renal nerve activation on RBF and the GFR are in the same direction, the magnitude of the reduction is different. Experimental studies in several animal models have indicated that the reduction in RBF is much greater (15% to 20%) than the reduction in the GFR (2% to 5%).
Another important issue relates to the structural changes of renal sympathetic activation or denervation on the renal micro- and macrovasculature. Renal sympathetic nerve activity seems to affect the vascular structure of renal vessels. The stimulation of a 1 adrenergic receptors by noradrenaline induces mitogen-activated protein kinase activation, suggesting that renal sympathetic nerves are implicated in the growth and hypertrophy of vascular smooth muscle cells. Experimental data suggest that the renal vessel wall-to-lumen ratio is greater in hypertensive rats, indicating a trophic effect of renal sympathetic activation on the arteriolar wall. The chemical destruction of peripheral sympathetic nerves with 6-hydroxy-dopamine significantly reduced the wall-to-lumen ratios in these animals, pointing to a favorable vessel remodeling with RSD.
Afferent renal sympathetic nerves originate mostly from the renal pelvic wall. Mechanoceptors respond to stretch, and chemoreceptors detect renal ischemia. The cell bodies of renal afferent nerves lie in the ipsilateral dorsal root ganglia (T6-L4). From there, ascending signals travel to centers in the central nervous system, mainly at the hypothalamic area, evoking functional changes. The hypothalamic paraventricular nucleus seems to play an important role in the autonomic control of the cardiovascular system. Renal afferents function as sensors of renal injury and stimulate sympathetic centers in the brain, thus increasing SNS activity and consequently blood pressure levels. Indeed, experimental studies in several models of renal injury (reduced renal mass by 5/6 nephrectomy, local phenol injection, 1-kidney 1-clip hypertension) point toward an important role of renal afferent nerves; the increased sympathetic activity in the posterior hypothalamus and elevated blood pressure levels were completely prevented by section of the renal afferents (dorsal rhizotomy). In contrast, afferent renal denervation by dorsal rhizotomy either does not prevent the onset and development of hypertension in spontaneously hypertensive rats or even results in a significant increase of mean arterial pressure while on a high-salt diet. Similar findings of salt-sensitive hypertension were observed with chemical afferent denervation induced by capsaicin. However, these findings seem of little clinical significance, because the net result of renal denervation is a marked reduction of blood pressure levels. The effects of low- and high-salt diets after RSD must be investigated to adequately address these concerns.
Historical Perspective of Sympathetic Denervation
Before the discovery of effective drug therapy, malignant hypertension was a devastating disease with a 5-year mortality rate of almost 100%. Thus, interventional approaches have been tested for its treatment. Sympathectomy has been applied mainly in patients with severe or malignant hypertension, as well as in patients with cardiovascular deterioration despite relatively good blood pressure reduction by other means. After the introduction of antihypertensive drugs, sympathectomy was reserved for patients who failed to respond to antihypertensive therapy or could not tolerate it.
Total sympathectomy was found to be impractical and poorly tolerated by humans. Sympathectomy had to include the abdominal organs to be effective, and thus the term “splanchnicectomy” was used. A much extended operation was performed in the early years of sympathectomy, which was later replaced by a more conservative surgery, from the 8th to the 12th dorsal vertebra. Sympathectomy was performed in either 1 or 2 stages, required a prolonged hospital stay (2 to 4 weeks) and a long recovery period (1 to 2 months), and more importantly required a very competent surgeon to perform it. It was thus performed only in a few selected centers in the United States (Boston, Michigan, Cleveland, Rochester, Miami, California) and Europe.
Several studies in patients with severe or malignant hypertension have confirmed that splanchnicectomy was associated with higher survival rates than “primitive” drug therapy, while the survival rates compared with untreated patients were tremendously higher. Characteristically, in a large observational study of >2,000 patients (1,506 who underwent splanchnicectomy), survival rates were more than doubled in patients who underwent sympathectomy, and the benefits were evident in all stages of hypertension. A satisfactory blood pressure response was observed in about half of the patients who underwent splanchnicectomy. In addition, sympathectomy rendered blood pressure more sensitive to antihypertensive drugs, allowing a reduction in the number and doses of administered drugs.
Adverse events were common, annoying, and some of them serious and included orthostatic hypotension, orthostatic tachycardia, palpitations, breathlessness, anhidrosis, cold hands, intestinal disturbances, loss of ejaculation, sexual dissatisfaction, thoracic duct injuries, and atelectasis. The advent of effective antihypertensive therapy, the impressive results of the Veterans Affairs studies that confirmed the benefits of drug treatment, and the significant adverse effects of splanchnicectomy changed therapeutic strategies, leading sympathectomy to the shadows, negligence, and disregard.
Historical Perspective of Sympathetic Denervation
Before the discovery of effective drug therapy, malignant hypertension was a devastating disease with a 5-year mortality rate of almost 100%. Thus, interventional approaches have been tested for its treatment. Sympathectomy has been applied mainly in patients with severe or malignant hypertension, as well as in patients with cardiovascular deterioration despite relatively good blood pressure reduction by other means. After the introduction of antihypertensive drugs, sympathectomy was reserved for patients who failed to respond to antihypertensive therapy or could not tolerate it.
Total sympathectomy was found to be impractical and poorly tolerated by humans. Sympathectomy had to include the abdominal organs to be effective, and thus the term “splanchnicectomy” was used. A much extended operation was performed in the early years of sympathectomy, which was later replaced by a more conservative surgery, from the 8th to the 12th dorsal vertebra. Sympathectomy was performed in either 1 or 2 stages, required a prolonged hospital stay (2 to 4 weeks) and a long recovery period (1 to 2 months), and more importantly required a very competent surgeon to perform it. It was thus performed only in a few selected centers in the United States (Boston, Michigan, Cleveland, Rochester, Miami, California) and Europe.
Several studies in patients with severe or malignant hypertension have confirmed that splanchnicectomy was associated with higher survival rates than “primitive” drug therapy, while the survival rates compared with untreated patients were tremendously higher. Characteristically, in a large observational study of >2,000 patients (1,506 who underwent splanchnicectomy), survival rates were more than doubled in patients who underwent sympathectomy, and the benefits were evident in all stages of hypertension. A satisfactory blood pressure response was observed in about half of the patients who underwent splanchnicectomy. In addition, sympathectomy rendered blood pressure more sensitive to antihypertensive drugs, allowing a reduction in the number and doses of administered drugs.
Adverse events were common, annoying, and some of them serious and included orthostatic hypotension, orthostatic tachycardia, palpitations, breathlessness, anhidrosis, cold hands, intestinal disturbances, loss of ejaculation, sexual dissatisfaction, thoracic duct injuries, and atelectasis. The advent of effective antihypertensive therapy, the impressive results of the Veterans Affairs studies that confirmed the benefits of drug treatment, and the significant adverse effects of splanchnicectomy changed therapeutic strategies, leading sympathectomy to the shadows, negligence, and disregard.
Current Use of Renal Sympathetic Denervation
RSD presents several significant advantages over the radical sympathectomy that was performed 5 decades ago. It is a localized procedure, it is minimally invasive, it has no systematic side effects, and its procedural and recovery times are very short.
Catheter-based RSD was studied in 50 patients with resistant hypertension, with 45 of them fulfilling anatomic eligibility criteria. RSD was achieved by radiofrequency ablation through the percutaneous insertion of a modified catheter (Symplicity; Ardian, Inc., Palo Alto, California). The efficacy of RSD was confirmed in a subgroup of 10 patients by the use of the very elegant and demanding noradrenaline spillover technique. Renal sympathetic ablation resulted in impressive blood pressure reductions that were maintained during the 12-month follow-up period ( Table 1 ). Five patients who were ineligible for the study for anatomic reasons were used as controls; blood pressure in these patients was gradually increased during the follow-up period.
| Variable | Systolic Blood Pressure (mm Hg) | Diastolic Blood Pressure (mm Hg) |
|---|---|---|
| Baseline (n = 45) | 177 ± 20 | 101 ± 15 |
| Reduction at 1 month (n = 41) | 14 (4) ⁎ | 10 (3) ⁎ |
| Reduction at 3 months (n = 39) | 21 (7) ⁎ | 10 (4) ⁎ |
| Reduction at 6 months (n = 26) | 22 (10) ⁎ | 11 (5) ⁎ |
| Reduction at 9 months (n = 20) | 24 (9) ⁎ | 11 (5) ⁎ |
| Reduction at 12 months (n = 9) | 27 (16) ⁎ | 17 (11) † |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


