Renal Artery Stent Placement
Christopher J. White
Atherosclerotic renal artery stenosis (RAS) is more common than was previously thought. In a screening study of 834 free-living Medicare patients, RAS of >60% was found in 6.8% of these patients through the use of ultrasound (1). In this sample of patients with an average age of 77.2 years, those with significant (>60%) RAS were more likely to be male (9.1%) than female (5.5%, p = .053), and have unilateral (88%) compared to bilateral (12%) involvement. Multivariate predictors of RAS were increasing age, high density lipoprotein (HDL) levels of <40 mg/dL, and increasing systolic blood pressure. RAS is the most common cause of secondary hypertension in the general population of hypertensive patients (2). In several clinical subsets of patients, RAS is more common, such as those patients with uncontrolled hypertension and renal insufficiency, and in patients with associated atherosclerotic coronary or peripheral vascular disease (3). In patients with renal insufficiency, the incidence of unsuspected RAS was 24% (4). In patients undergoing coronary angiography for suspected coronary artery disease, the incidence of RAS ranges from 15% to 23% (Table 19.1) (5, 6, 7, 8, 9). In patients with abdominal aortic aneurysms or peripheral arterial occlusive disease, unsuspected RAS may be present in approximately one-third of patients (10,11).
General agreement concurs that the natural history of RAS is for lesions to progress over time. The incidence of renal artery lesion progression in angiographic serial studies ranges from 39% to 49% (12, 13, 14, 15, 16). The risk of lesion progression is related to the severity of the stenosis (Fig. 19.1) (17). The progression of RAS from a nonobstructive lesion to a greater than 75% stenosis was associated with loss of renal function (12).
In a prospective study of patients with medically treated RAS, progression occurred in 42% of patients over a 2-year period (11% progressed to occlusion) (18). In a recent trial of modern medical therapy compared to interventional therapy for renovascular hypertension, 16% of the medically treated patients suffered renal artery occlusion at 1 year (19). Of particular importance is the recognition that the progression of RAS and loss of renal function occur despite control of blood pressure (20).
INDICATIONS FOR RENAL ARTERY REVASCULARIZATION
Patients with hemodynamically significant RAS and uncontrolled hypertension, renal insufficiency, or a cardiac disturbance syndrome (e.g., flash pulmonary edema) are appropriate candidates for revascularization. Patients starting hemodialysis and who have viable renal parenchyma supplied by stenotic renal arteries, and those with RAS and refractory congestive heart failure or unstable angina, also should be considered candidates for revascularization (21, 22, 23, 24, 25, 26).
Three clinical indications are generally accepted for renal artery revascularization when a hemodynamically significant RAS is present. The first is poorly controlled hypertension on adequate medical therapy (two or more antihypertensive drugs), or in a patient intolerant of hypertensive medications. The second indication is to preserve renal function. The third indication is to treat cardiac disturbance syndromes, such as acute coronary ischemia or congestive heart failure (“flash” pulmonary edema), which are exacerbated by renovascular hypertension.
The treating physician should have a high clinical suspicion that the target RAS is causally related to the clinical
symptoms. This determination should be based upon both functional and anatomic assessments of the RAS. Functional assessments include an estimation of the severity of the stenosis from pressure gradients either directly measured with catheters or pressure wires, or noninvasively assessed by Doppler ultrasound signals. Other functional assessments include a measurement of the kidney size and a determination of renal function or perfusion through radionuclide methods or clinical chemistry tests, such as selective renal vein renin and creatinine clearance determinations. Anatomic assessments include visualization of the diameter or area stenosis by noninvasive tools, such as computed tomographic angiography (CTA), magnetic resonance angiography (MRA), or ultrasound imaging. Invasive imaging options include intravascular ultrasound (IVUS) and the gold standard, angiography (Fig. 19.2).
symptoms. This determination should be based upon both functional and anatomic assessments of the RAS. Functional assessments include an estimation of the severity of the stenosis from pressure gradients either directly measured with catheters or pressure wires, or noninvasively assessed by Doppler ultrasound signals. Other functional assessments include a measurement of the kidney size and a determination of renal function or perfusion through radionuclide methods or clinical chemistry tests, such as selective renal vein renin and creatinine clearance determinations. Anatomic assessments include visualization of the diameter or area stenosis by noninvasive tools, such as computed tomographic angiography (CTA), magnetic resonance angiography (MRA), or ultrasound imaging. Invasive imaging options include intravascular ultrasound (IVUS) and the gold standard, angiography (Fig. 19.2).
TABLE 19.1. PREVALENCE OF RENAL ARTERY STENOSIS (RAS) AT THE TIME OF CARDIAC CATHETERIZATION | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
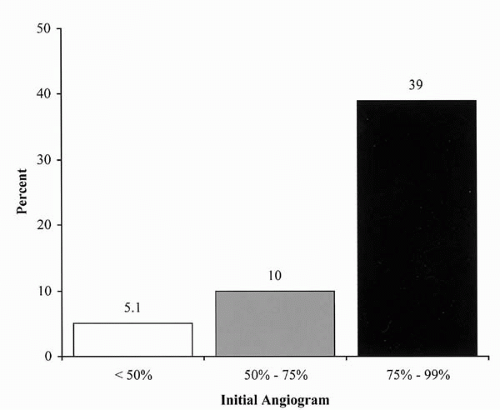 Figure 19.1. Progression of renal artery stenosis to occlusion over 13 months and the relationship to the baseline stenosis (15). |
PATIENT SELECTION
Appropriate patient selection requires clinical judgment and an integration of functional and anatomic information. The treating physician should consider carefully all risks, potential benefits, and alternative treatment strategies. The criteria used to determine the anatomic severity of an RAS continues to evolve. It is generally accepted that an angiographic RAS of <50% does not require revascularization, whereas a stenosis of ≥70%, in general, merits revascularization. Hemodynamic criteria for renal intervention include a translesional systolic pressure gradient of ≥20 mm Hg or a mean gradient of ≥10 mm Hg (27).
Patients being considered for revascularization for uncontrolled hypertension should have failed at least two and preferably three antihypertensive medications that work well together. Patients with renal insufficiency and a hemodynamically significant stenosis are candidates for renal artery revascularization. Patients who manifest renal insufficiency despite angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARB) are candidates for renal revascularization if they have (a) bilateral renal artery stenoses, (b) unilateral stenosis with a solitary kidney, or (c) unilateral stenosis with severe dysfunction of the contralateral kidney. Finally, patients with cardiac disturbance syndromes due to renovascular hypertension or volume overload are candidates for the revascularization of hemodynamically significant renal artery stenoses.
PERCUTANEOUS RENAL ARTERY REVASCULARIZATION
Percutaneous transluminal renal balloon angioplasty (BA) is the treatment of choice for RAS caused by fibromuscular dysplasia (FMD) (28,29). However, BA is associated with a lower primary success rate for atherosclerotic lesions and a restenosis rate of approximately 50% over 6 months (30,31).
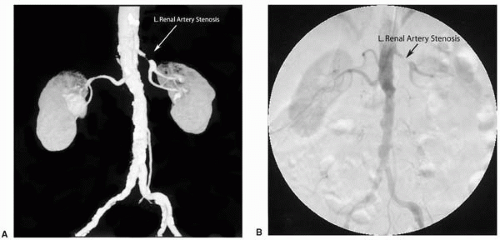 Figure 19.2. (A) Comparison of renal CTA and (B) aortogram showing a critical left renal artery stenosis. CTA: computed tomographic angiography. |
The Dutch Renal Artery Stenosis Intervention Cooperative (DRASTIC) trial was a poorly planned attempt to determine the efficacy of medical therapy compared to balloon angioplasty or percutaneous transluminal renal angioplasty (PTRA) for blood pressure control in patients with renovascular hypertension (19). An advantage was observed for the PTRA group at 3 months, but the intention-to-treat analysis at 1 year was invalidated by a 44% crossover of medical treatment patients to PTRA (Fig. 19.3).
Atherosclerotic aorto-ostial renal artery lesions are particularly difficult to treat with balloon dilatation alone, because they are prone to restenosis due to vascular recoil caused by confluent plaque from the aorta flowing into the renal artery. For this reason, atherosclerotic aorto-ostial renal stenoses are considered unsuitable lesions for BA alone (31,32).
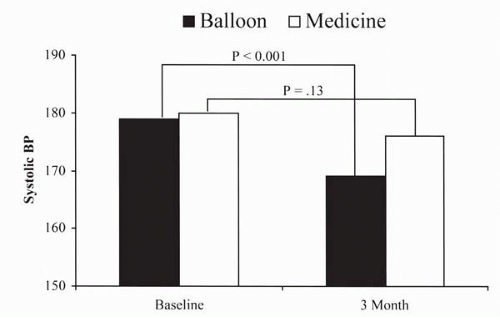 Figure 19.3. Blood pressure results after 3 months (before crossover) comparing balloon angioplasty to medical therapy for renovascular hypertension (19). |
A comparison of balloon versus stent angiographic and hemodynamic outcomes has been assessed in atherosclerotic RAS (33). Quantitative vascular angiography (QVA) and translesional pressure gradients were measured in 18 patients serving as their own controls. Stents were more effective than BA in these atherosclerotic renal artery lesions (Table 19.2).
A randomized controlled trial comparing BA to stent placement for aorto-ostial atherosclerotic RAS in patients with renovascular hypertension demonstrated that stents are superior to balloon dilatation alone (34). A total of 42 patients and 51 arteries were randomized to BA, and 42 patients and 52 arteries were randomized to stents. The procedure success and long-term patency markedly favored the stent group (Table 19.3). Over the course of the study, 12 (29%) patients in the balloon group required stents. This large number of crossover patients confounded the analysis of the clinical endpoint at 1 year. Using the intention-to-treat analysis, no difference was found for blood pressure
control, renal function, or complications between the two groups.
control, renal function, or complications between the two groups.
TABLE 19.2. BALLOON VERSUS STENT (33) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
TABLE 19.3. BALLOON VERSUS STENT RANDOMIZED CONTROLLED TRIAL (34) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


