Renal Arteries
Peter Lanzer
Ralf Weser
Renal Artery Disease
Renal artery diseases comprise a wide range of systemic and local disorders that may affect directly or indirectly both the large and small vessels of the kidneys. The most frequent cause of renal artery diseases is atherosclerosis, followed by fibromuscular dysplasia. Less frequent causes include vasculitides such as Takayasu’s arteritis, arteriovenous malformations, aneurysms, renal venous diseases, extrinsic vascular compression or damage by cysts or tumors, renovascular radiation injuries, neurofibromatosis lesions, retroperitoneal fibrosis, thromboembolic disease, traumata, and a number of renal parenchymal diseases. Several diseases, notably diabetes mellitus and systemic hypertension, may affect both the renal parenchyma and the renal vasculature at the same time (for review, see references 1 and 2).
Renal Artery Stenosis
Renal artery stenosis (RAS) appears to be a common cause of renovascular hypertension (RVH), hypertensive nephropathy (HTN), ischemic nephropathy (IN), and renal insufficiency, including end-stage renal disease (ESRD) (for review, see reference 3).
The recognition that RAS and systemic hypertension4,5 were associated with each other, as were RVH and activation of the renin-angiotensin-aldosterone system (RAAS),6 was an important step toward understanding the pathophysiologic links between renovascular disease and systemic hypertension. RAS-related reduction in renal perfusion, autoregulatory vasodilatation of the afferent (precapillary) arterioles (mediated by tubuloglomerular feedback and by direct myogenic responses with consecutive renin release triggering RAAS activation), angiotensin II release leading to vasoconstriction of the efferent (postcapillary) arterioles, an increase in intraglomerular pressure, and the systemic effects of RAAS activation have become widely recognized as critical pathogenetic principles involved not only in regulation of renal perfusion but also in preservation of glomerular filtration and development of RVH. Thus, sustained hypertension may induce structural renovascular changes triggering a vicious circle that may result in the development of HTN.7 Nevertheless, the relationship between the severity of RAS and the degree of blood pressure elevation does not appear to be linear8 and the specific factors responsible for the development of HTN have yet to be identified.
RAS may also cause global chronic renal ischemia, which, if sustained, may result in progressive interstitial fibrosis, and the glomerular and intrarenal vascular nephrosclerosis associated with IN (for review, see reference 9). However, the relationship between the severity of RAS and IN remains uncertain. On the basis of the definition of critical ischemia, RAS covering more than 70% to 80% of the cross-sectional area (or 45% to 56% of the diameter) has been considered hemodynamically significant.10 Besides causing global chronic renal ischemia, atherosclerotic RAS (ARAS) also appears to be a potential source of atheroembolism causing regional renal ischemic defects and microrinfarcts; this has been documented by histology in patients with IN.11 However, the importance of thromboembolism associated with RAS in the pathogenesis of IN still remains to be elucidated.
Finally, RAS appears to be associated with chronic renal insufficiency defined as renal failure (glomerular filtration rate, GFR 5 to 25 mL/min) and ESRD (GFR <5 mL/min) (for review, see references 9 and 12). Yet, chronic renal insufficiency represents a common final pathway of numerous disorders including hypertension, diabetes, interstitial nephritis, glomerulonephritis, acute tubular necrosis, immune-related
disorders, and tubulopathies, and the specific relevance of RAS has yet to be explored. Interestingly, systematic renal biopsies performed in a well-defined population of patients with ESRD showed that hypertension-ischemia-induced nephropathy was the most frequent etiology (12.9%) followed by diabetic (10.4%), immunoglobulin-A (IgA) (9.1%), and thin-membrane (8.5%) renal diseases.13
disorders, and tubulopathies, and the specific relevance of RAS has yet to be explored. Interestingly, systematic renal biopsies performed in a well-defined population of patients with ESRD showed that hypertension-ischemia-induced nephropathy was the most frequent etiology (12.9%) followed by diabetic (10.4%), immunoglobulin-A (IgA) (9.1%), and thin-membrane (8.5%) renal diseases.13
In most cases, RAS is caused by renal artery atherosclerosis (up to 90% of proximal RAS) and renal artery fibromuscular dysplasia (up to 10% of proximal RAS).
Atherosclerotic Renal Artery Stenosis
Atherosclerotic renal artery stenosis (ARAS) is a frequent manifestation of systemic atherosclerosis,14 yet its true incidence and prevalence remain uncertain. In a recent study the estimated incidence of ARAS in people 65 years of age and older was 6.8%15 and it was substantially higher in patients with atherosclerotic disease in other vascular beds (for review, see reference 16).
Although the causal relationship between ARAS and systemic hypertension, HTN, IN, and chronic renal insufficiency including ESRD appears likely, still much has to be learned about the true nature of the assumed pathogenetic links in individual patients. For example, it is well-recognized that RAS may or may not be associated with hypertension; in fact, in one of the early studies hypertension was present only in 50% of patients presenting with RAS.17 Similarly, the severity of RAS does not appear to correlate with the degree of the associated renal dysfunction.18 Some of the variability could be related to differences in biological dignity of atherosclerotic lesions underlined to ARAS, expressed for example in the embolic activity, biological and mechanical stability, and other factors. In addition, the frequent anatomic variants in renal blood supply (see below) and special renal hemodynamics could modify the functional impact, including:
High blood flow rates: The kidney receives approximately 1.0 to 1.2 L blood/min, corresponding to approximately 20% of the resting cardiac output; this makes the kidney the organ with the highest blood flow per gram of tissue of the body (approximately 400 mL/min/100 g tissue vs., e.g., liver with 20 mL/min/100 g tissue)
Low oxygen extraction rates: For example, oxygen delivery is approximately 84.0 mL/min/100 g, while oxygen consumption is approximately 6.8 mL/min/100 g; this corresponds to an extraction rate of 8.1%
High oxygen demand: At a rate of 11.9 O2 mL/min/100 g, this is second only to the heart
High glomerular capillary pressure compared with other capillary beds in the body: 60 versus 13 mm Hg;
Precise autoregulation 19
Near maximum flow conditions at rest implying near maximum peripheral dilatation: This corresponds to an approximately twofold flow reserve compared with an approximately 20-fold flow reserve in skeletal muscle (see Chapter 2).
Therefore, it appears likely that the severity of ARAS may be modified by numerous other factors acting in concert and participating in the pathogenesis of endorgan damage and injury. Thus, pathogenetic synergism between thromboembolic events and sustained hypercholesterolemia has been previously discussed.20
ARAS usually involves the proximal third of the artery, frequently (in about 75% of cases) including the ostia. In up to 30% of cases, both renal arteries may be stenosed. In severe cases more distal arterial segments may also be narrowed. The reason for the susceptibility of the ostia to atherosclerosis has not been clarified, yet it may be related to altered mechanical properties due to differences in the histological architecture of the transitional zone between the elastic aorta and muscular renal artery.21 The rate of progression of ARAS is a matter of debate. In one study using duplex classification of renal stenosis severity, the cumulative rates of progression from normal to <60% narrowing at 1, 2, and 3 years were 0%, 0%, and 8%, and those from <60% to >60% were 30%, 44%, and 48%, respectively.22 Similar rates of progression were reported more recently, with the risk of progression being highest in individuals with a pre-existing RAS in either one of the renal arteries, elevated systolic blood pressure, and diabetes.23 Progression of ARAS appears to be associated with a progressive loss of renal tissue.24
Fibromuscular Dysplasia
Fibromuscular dysplasia (FMD), first described in 1938,25 is a vascular disease of unknown origin that most frequently affects the renal arteries (in 60% to 75% on one side only, in 35% bilaterally) and internal carotid arteries (25% to 30%). However, any artery and, rarely, even veins may be involved. FMD predominates in women (female-to-male ratio 3:1), and the typical age of presentation is 25 to 50 years. Hypertension, thromboembolism, and hemorrhage are typical presenting symptoms. Depending on the location of FMD lesions, medial, perimedial, intimal, and adventitial disease types may be distinguished (for review, see references 26 and 27).
On the basis of appearance, three angiographic types have commonly been distinguished:
Type 1 angiography is described as a “string of beads,” affects about 80% of cases, and mostly represents medial FMD. This type reveals a typical beading pattern with alternating segments of strictures and dilations, most frequently located in the middle-to-distal segment of the renal artery. The dilated segments are larger than the nominal diameter of the vessel. The differential diagnosis includes atherosclerotic disease, arteritis, and vasospasms.
Type 2 angiography is “diffuse,” affecting about 10% of cases, and mostly involves intimal but also perimedial locations. It presents as a long tubular stenosis. The differential diagnosis includes dissections, arteritis, congenital dyplasias, compression from outside, vasospasm, and functional narrowing due to decreased flow secondary to proximal or distal stenotic lesions.
Type 3 angiography is described as “solitary,” affecting about 5% of cases, and is frequently intimal FMD. It is associated with focal concentric lesions that may be difficult to distinguish from atheroma or pseudoaneurysm by angiography.
Diagnosis of renaL artery stenosis
Clinical findings suggestive of RAS include:
Sudden onset or sudden worsening of a severe and refractory hypertension28
Rapid elevation of serum creatinine, particularly if associated with institution of angiotensin-converting enzyme
(ACE) inhibitor treatment (due to the abolition of compensatory vasoconstriction of the efferent arterioles)29
Hypertension associated with small kidneys
Different-sized kidneys
Hypertension in patients with severe systemic atherosclerosis30
Recurrent flash pulmonary edema31
In addition, hyponatremia may be more common.32 However, in the majority of cases RAS remains clinically silent.
According to the recent ACC/AHA guidelines,16 diagnostic evaluations for RAS are indicated in patients with the onset of hypertension before the age of 30 years, patients with the onset of severe hypertension after the age of 55 years (both class I evidence level B), those with accelerated hypertension or drugresistant hypertension or malignant hypertension (class I evidence level C), patients with azotemia or worsening renal function associated with administration of ACE inhibitors, unexplained renal atrophy or size difference between the two kidneys >1.5 cm, and finally those with unexplained “flush” pulmonary edema (all class I evidence level B). Class IIa evidence level B indications include patients with unexplained renal failure, and Class IIb evidence level B and C concerns patients with multivessel coronary artery disease or peripheral arterial disease at the time of arteriography and patients with unexplained congestive heart failure or refractory angina, respectively.
Noninvasive screening for RAS includes duplex ultrasonography, magnetic resonance angiography, and CT angiography (all ACC/AHA class I evidence level B).16 Given a high level of suspicion or inconclusive noninvasive tests, selective arterial digital subtraction angiography (DSA) is recommended to establish the diagnosis (ACC/AHA class I evidence level B). Despite progress in noninvasive imaging methods, DSA still represents the gold standard and reference technique to provide a definitive RAS diagnosis and to allow an anatomically precise road map for revascularization (for review, see reference 33). A captopril renogram, for which 25 to 50 mg captopril PO is administered before radioisotope scanning and usually using 99mTc-DTPA as a tracer, may allow identification of an angiotensin-II-dependent kidney; in such cases, following captopril administration there is a decrease in GFR (perfusion decreased by >40%), a delayed peak uptake in the ipsilateral kidney, and—frequently—enhanced compensatory perfusion in the contralateral kidney.34,35 However, because of the rather low sensitivity and specificity, captopril renography has been largely abandoned. Similarly, measurements of renin plasma activity at baseline and following captopril challenge,36 side-selective renal vein renin determinations where the ipsilateral kidney stimulates renin production while the contralateral kidney suppresses it37 are rarely employed.
In peri-interventional settings, DSA represents the only method allowing reliable assessment of the lesion and of the progress of the intervention.
Peri-interventional RAS DSA should unequivocally determine the severity and morphology of the target lesion and the complete ipsilateral renal blood supply. The anatomy of the renal artery and its branches, clinical x-ray anatomy of the variants, the renal collateral circulation (capsular, peripelvic, and periureteric systems),38,39 and the principles of diagnostic renal arteriography have been covered extensively in the literature.40
Radiographic evaluation of RAS consists of abdominal aortic DSA and, if needed, selective renal DSA, both performed as breath-hold imaging (shallow inspiration). First, the pigtail catheter is positioned just above or at the level of the presumed take-off of the renal arteries (lumbar vertebra L1 or L2, right higher than left). Then, a DSA sequence is taken that is long enough to cover at least the arterial but preferably also the nephrogenic and venous phases of contrast-agent passage using an automatic injector. In evaluating the arterial phase, the origin, number, size, course, and branching pattern of the renal arteries are determined. Usually the main renal artery, its segmental branches, and the interlobar and arcuate arteries can be differentiated. Interlobular arteries and arterioles, however, cannot be clearly distinguished. In the nephrogenic phase, the cortical arteriogram, glomerulogram, cortical nephrogram, and complete nephrogram are displayed in a short sequence, with a complete washout of contrast agent taking 20 seconds and
longer. The venous phase partially overlaps the nephrogenic phase, starting about 3 seconds after the entry of the contrast agent into the renal artery and peaking at about 8 seconds. Because of the overlapping vascular structures, intrarenal vein structures can barely be distinguished and only some parts of the extrarenal veins can be evaluated.40 Figure 11-1 shows pattern variations in the ramification of renal arteries. In Figure 11-2, the principal collateral pathways of the renal artery are shown.
longer. The venous phase partially overlaps the nephrogenic phase, starting about 3 seconds after the entry of the contrast agent into the renal artery and peaking at about 8 seconds. Because of the overlapping vascular structures, intrarenal vein structures can barely be distinguished and only some parts of the extrarenal veins can be evaluated.40 Figure 11-1 shows pattern variations in the ramification of renal arteries. In Figure 11-2, the principal collateral pathways of the renal artery are shown.
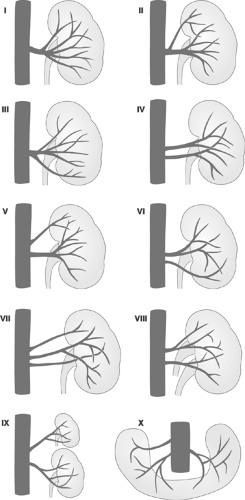 FIGURE 11-1. Variations of patterns of ramification of renal arteries. (Modified from Lusza G. X-ray Anatomy of the Vascular System. Philadelphia: JB Lippincott, 1964:227-231.) |
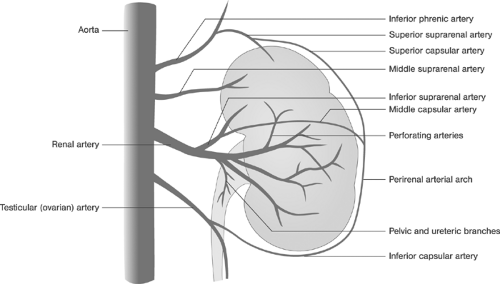 FIGURE 11-2. Intrarenal and extrarenal collateral circulation of the renal artery. (Modified from Lusza G. X-ray Anatomy of the Vascular System. Philadelphia: JB Lippincott, 1964:227-231.) |
In patients with suspect anatomy in the overview DSA aortograms, side-selective renal arteriograms using preshaped catheters are required. Because of the vulnerability of the ostia care must be taken to avoid injury, and gentle “puff” injections of the contrast agent are used for localization of the ostia. Because of the variable laterality of the origin of the renal arteries and variable diameter of the abdominal aorta, different oblique angulations may be required in individual patients to visualize the true profile of the ostia and of the proximal segment free of overlap with the adjacent aorta. Based on extensive CT-based measurements, anteroposterior (AP) and 20-degree left anterior oblique (LAO) projections (combined successfully in 86% of cases) for the left renal artery and 20-degree LAO, 40-degree LAO, and AP projections (combined successfully in 93% to 95% of cases) for the right renal artery have been recommended. The largest yield of orthogonal ostial projections on either side was achieved using a 20-degree LAO projection. Deviation from the optimal degree of angulation by ±10% may be responsible for 5-mm proximal foreshortening, thus hiding the ostium from adequate visualization. On the basis of this data, the combination of AP, 20-degree, and 40-degree LAO projections allows adequate angiographic definition of both ostia in both genders in the vast majority (92%) of all individuals.41
In patients with RAS, the location, severity, and morphology of the target lesion should be documented. Typically, ostial stenoses (≤5 mm from the orifice), nonosteal stenoses (>5 mm from the orifice), branch stenoses, and angiographic lesions of less than and at least 50% diameter are distinguished. The degree of RAS can be calculated using the edge detection or densitometry-based quantitative angiography programs as 1 minus the ratio of the diameter of the lumen at the stenosis to the diameter of the lumen of the uninvolved renal artery distal to the stenosis multiplied by 100. Stenoses of at least 50% diameter are considered hemodynamically significant. In addition, the minimal luminal diameter (MLD), measured in millimeters across the stenosis, should be reported.42
In patients with angiographically indeterminate or borderline lesions (typically 50% to 70% diameter stenoses), translesional blood-pressure measurements may be required. However, if pressure wire has not been used, invasive measurements require crossing the lesion with the guidewire and usually a curved catheter with the attending risk of injury; for this reason, invasive pressure measurements should be performed with care and only in selected cases. In the absence of any documented evidence, some investigators consider a 20 mm Hg peak-to-peak systolic pressure gradient between the abdominal aorta and distal renal artery to be hemodynamically relevant, whereas others consider a resting peak systolic pressure gradient of 10 mm Hg at baseline conditions or a pressure gradient of 20 mm Hg after vasodilatation significant (for review, see reference 43). These and other threshold values have been considered as indications for revascularization in the presence of borderline lesions. Translesional gradients should preferably be measured using 0.0014-in. pressure wires; however, in current clinical practice, the majority of measurements are performed using two different fluid-filled catheters with the tip at the ostium and distal to the lesion, respectively. The accuracy, that is, proximity to the true value, and precision, that is, reproducibility of measurements, depend on a number of factors including the coaxial position of the tip, the luminal diameters of the catheters,
the position of the orifices (end-hole catheters measure higher intraluminal pressure because of additional conversion of the kinetic energy into pressure), and the catheter material (for review, see reference 43). To obtain reliable data, standardized and carefully performed measurements are required.
the position of the orifices (end-hole catheters measure higher intraluminal pressure because of additional conversion of the kinetic energy into pressure), and the catheter material (for review, see reference 43). To obtain reliable data, standardized and carefully performed measurements are required.
The morphology of target RAS lesions has not yet been systematically evaluated, yet the presence of thrombus definitely increases the risk of distal embolization, and other lesion characteristics such as ulcerations probably deserve further study. In addition to the renal artery, evaluation of the ostium and the adjacent aortic walls is important. Angulation of the renal artery take-off, the degree of calcification, and the presence of plaques should be noted.
Since the risks of contrast-induced nephropathy (CIN) are greater in patients with pre-existing renovascular disease, strict adherence to the established standard of iodinated contrast agent administration is required. CIN can be defined as serum creatinine elevation of ≥0.5mg/dL (44 μmol/L) with baseline creatinine of ≥2.0 mg/dL (176 μmol/L), or as serum creatinine elevation of ≥1.0 mg/dL (88 μmol/L) with baseline creatinine of ≥2.0 mg/dL (176 μmol/L) occurring within 48 hours after contrast exposure44,45; however, other definitions are also available in the literature.
Recommendations for CIN prevention have recently been summarized and include:
Careful risk-benefit assessment, particularly in patients with pre-existing renal dysfunction
Avoidance of volume depletion, and adequate hydration with intravenous saline before and after the procedure (a note of warning: particular care must be taken here in patients with heart failure)
The use of low or iso-osmolal nonionic contrast agents (low-osmolar contrast media, LOCM)
The use of the lowest possible dose of the iodinated contrast agent
Avoidance of nephrotoxic agents
Oral administration of the antioxidant N -cetylcysteine (600 mg b.i.d.) on the day of angiography and the day after (for review, see references 46,47).
The benefits of administering other agents such as diuretics, calcium antagonists, or theophylline are questionable, and their use is not recommended. To reduce contrast agent exposure, carbon dioxide might be considered for diagnostic evaluations below the diaphragm, yet experience in peri-interventional settings has been limited.48 Suggested indications for invasive angiography in patients with RAS are summarized in Table 11-1.49 Suggested indications for RAS revascularization based on a traditional approach are provided in Table 11-2.50 In Table 11-3, predictors of clinical success with regard to renal function are summarized. Table 11-4 gives suggested indications for surgical RAS revascularization.
Revascularization of Renal Artery Stenosis
RAS has been treated surgically since 197351 and, at present, essentially three different surgical approaches of revascularization are available, namely, aortorenal bypass, thromboendarterectomy, and reimplantation (for review, see reference 52). Although the published short- and long-term results of RAS revascularization have been satisfactory (for review, see reference 53), the use of surgery for isolated RAS has rapidly declined with the availability of endovascular treatment options.
TABLE 11-1. Suggested Indications for Invasive X-ray Angiography for Evaluating Renal Artery Stenosis; Criteria Society of Interventional Radiology | |||
|---|---|---|---|
|
Renal artery angioplasty was introduced by Grüntzig et al. in 1978.54 In early series55,56 the reported technical success rates ranged between 73% and 100% in patients with FMD, and between 75% and 100% in patients with nonostial ARAS. The corresponding rates of medical cure and improvement were 25% to 63% and 13% to 63% for FMD, and 7% to 47% and 31% to 60% for nonostial ARAS, respectively (for review, see references 57 and 58). The introduction of stent-supported renal artery angioplasty in the early 1990s59 markedly improved the safety of the intervention and extended the spectrum of indications to include the treatment of ostial lesions.60 Thus, for example, in the series reported by Blum et al.,60 technical success (defined as residual stenosis of <50% according to angiography and a trans-stenotic pressure gradient of <20 mm Hg) was achieved in 96% of all treated lesions (71 of 74). At 5-year follow-up, a sustained improvement in mean arterial blood pressure and no significant changes in serum creatinine were observed. In published uncontrolled series, the combined success rate (cure or improvement of hypertension) was 65% to 80%, and in-stent restenosis ranging between 11% and 17% was reported. In addition, a variable improvement or stabilization of plasma creatinine concentration has been reported (for review, see reference 58). Selected early results of stent-supported renal artery angioplasty for RAS treatment are provided in Tables 11-5,11-6,11-7 and 11-861
Marked improvements in endovascular instrumentation have allowed downsizing of the interventional systems: 0.035-in. guidewires have been replaced by 0.0014-in. ones; bulky peripheral over-the-wire dilatation balloon catheters have been replaced by low-profile rapid-exchange balloon catheters; and stent delivery
systems with distal protection devices have been introduced.62 All these have transformed RAS revascularization into a less complex and safer procedure, similar to a coronary intervention. Accordingly, the coronary-like approach to RAS intervention can be defined by the use of small size introductory sheath, typically 6F, to reduce trauma, pre-shaped guiding catheters to improve seating and steering of the instrumentation, coronary 0.014-in. guidewires, low-profile dilatation balloon catheters, and/or stent-delivery devices with rapid-exchange design to facilitate each interventional step.
systems with distal protection devices have been introduced.62 All these have transformed RAS revascularization into a less complex and safer procedure, similar to a coronary intervention. Accordingly, the coronary-like approach to RAS intervention can be defined by the use of small size introductory sheath, typically 6F, to reduce trauma, pre-shaped guiding catheters to improve seating and steering of the instrumentation, coronary 0.014-in. guidewires, low-profile dilatation balloon catheters, and/or stent-delivery devices with rapid-exchange design to facilitate each interventional step.
TABLE 11-2. Suggested Indications for Renal Artery Stenosis (RAS) Revascularization Based on a Traditional Approach | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|



