Fig. 13.1
Large adenocarcinoma of the left kidney: preoperative embolization. (a) Arteriography: two arteries feed the left kidney, a main renal artery and an inferior accessory artery. (b) Selective injection of the inferior accessory artery, which clearly reveals that it also participates to the perfusion of the tumor. (c) Truncal embolization by coils, relatively distal to allow surgical ligation while accessing the pedicle
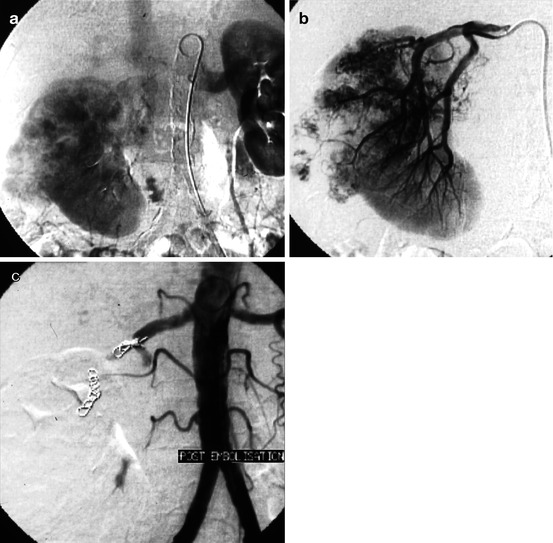
Fig. 13.2
Large adenocarcinoma of the right kidney: preoperative embolization, the morning before surgery. (a) Late phase of arterial opacification: patent renal vein, without abnormal arteriovenous communication, allowing the use of microparticles. (b) Selective catheterization to embolize with particles the branches feeding the tumor, followed by a proximal truncal occlusion. (c) Final aortographic control, sparing of few centimeters of the trunk of the patent right renal artery, in order to simplify surgical ligation during nephrectomy
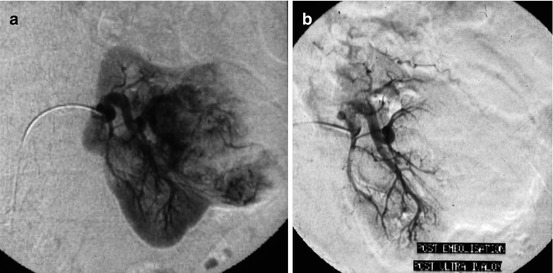
Fig. 13.3
87 years old, large adenocarcinoma of the left kidney judged as inoperable. Back pain + hematuria. A palliative embolization was planned. (a) Selective injection at the nephrographic phase: the tumor spars the lower pole of the kidney. A hyperselective embolization with particles was decided. (b) Arteriographic control after palliative tumoral exclusion by particles
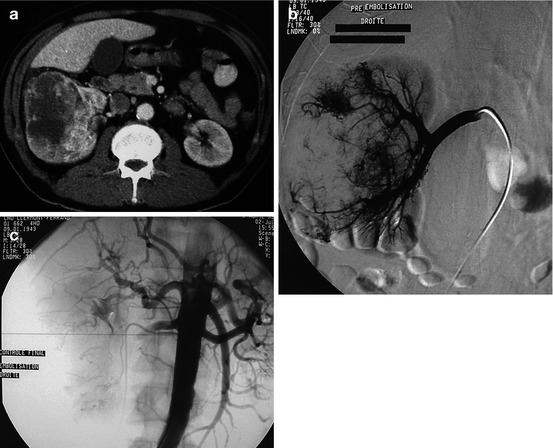
Fig. 13.4
Very large adenocarcinoma of the kidney with extension to the inferior vena cava and lung metastases, causing a severe deterioration of the general condition with fever, back pain, and hematuria. The patient was considered as inoperable. Palliative embolization was decided, in order to decrease the hematuria and the paraneoplastic syndrome. (a) CT: massive tumor involving the entire kidney with venous invasion. The left kidney is normal. (b) Selective injection of the right renal artery: almost all of the right kidney was involved, and a complete exclusion of this kidney was then performed (normal left kidney). (c) Control after exclusion with particles (microparticles of 500–700 and 900–1,200 μm)
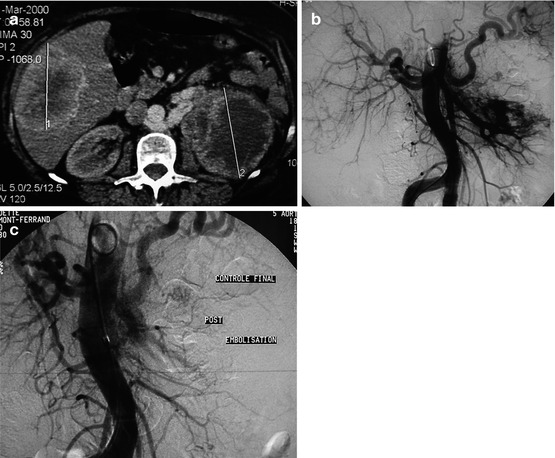
Fig. 13.5
Very large left renal tumor with liver metastases in a 75-year-old W with hematuria and polycythemia. (a) CT: renal tumor and liver metastasis; note a retroaortic patent left renal vein. (b) Aortography. (c) Control aortography after exclusion by microparticles
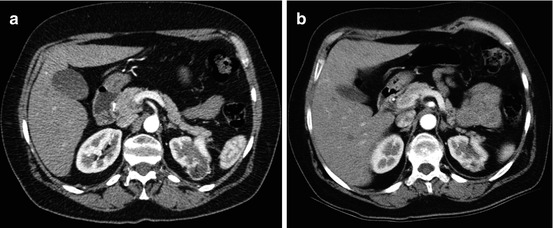
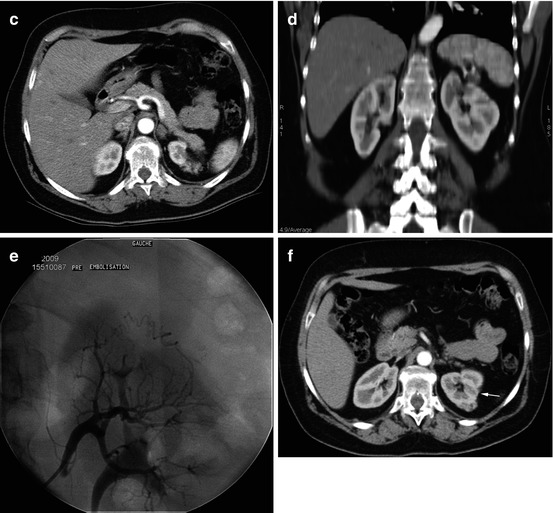
Fig. 13.6
Preventive embolization of an angiomyolipoma. (a) Asymptomatic 35 mm angiomyolipoma, discovered on a CT scan indicated for back pain in a 57-year-old M. Taking into account its size, imaging follow-up of this kidney was initially decided. (b–d) CT scan 1 year later: the exo-renal angiomyolipoma, measuring now 55 mm, is fed (coronal reconstruction) by a large arterial pedicle designated to the upper pole. Given this growth in size, an embolization was decided to prevent bleeding complication. (e) Selective arteriography: the vascularization of the angiomyolipoma was provided by upper pole branches that have been hyperselectively catheterized to exclude the tumoral territory by microparticles (100–300 and 300–500 μm in diameter). (f) CT scan 2 years later: note the retraction of the external cortex (arrow) and a small residual tumor (less than 15 mm in size), warranting further follow-up
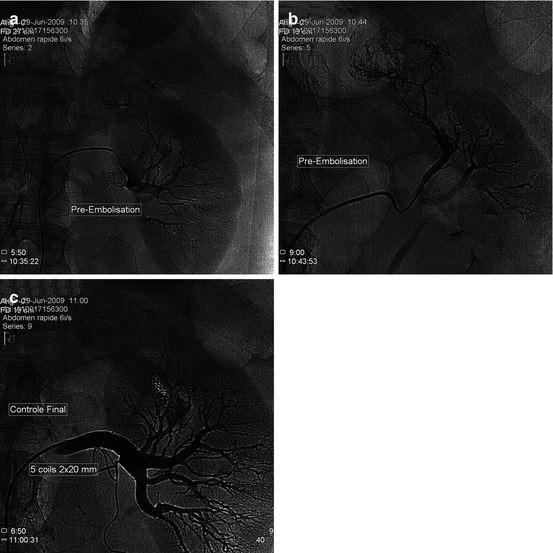
Fig. 13.7
Growth in size of an angiomyolipoma, measuring now 7 cm in a 65-year-old M: a preventive exclusion was decided. (a) Selective catheterization: tumoral hypervascularization with exo-renal development from the left upper pole. (b) Hyperselective injection. (c) Arteriographic control after selective exclusion with particles + coils
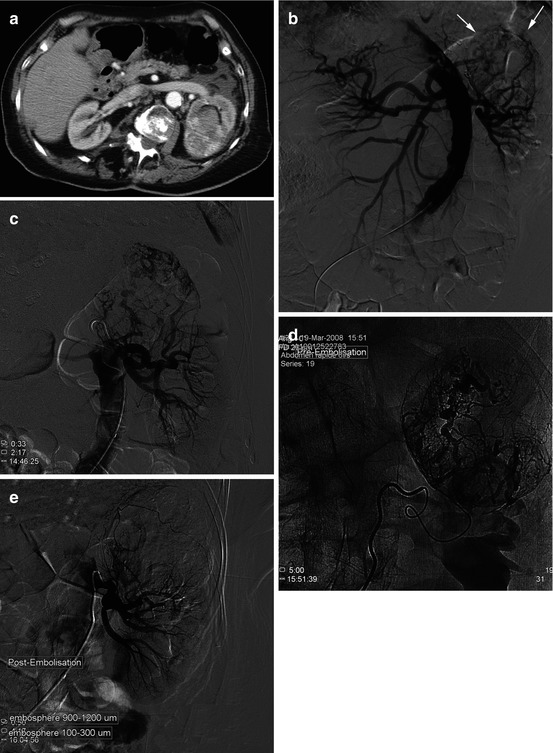
Fig. 13.8
Anticoagulation treatment for 6 months for thromboembolic disease; hospital admission for severe hematuria and left back pain. The assessment of hemostasis showed a PT = 27 %. (a) CT: large expansive process in which fat density was individualized: a hemorrhagic complication of an angiomyolipoma was suggested. (b) Aortography: the aorta and renal arteries are tortuous; hypervascular expansive process of the upper pole of the left kidney (arrow). (c) Selective renal artery injection. (d) Hyperselective catheterization using a microcatheter. (e) Selective renal arteriographic control after exclusion of the tumor by microparticles
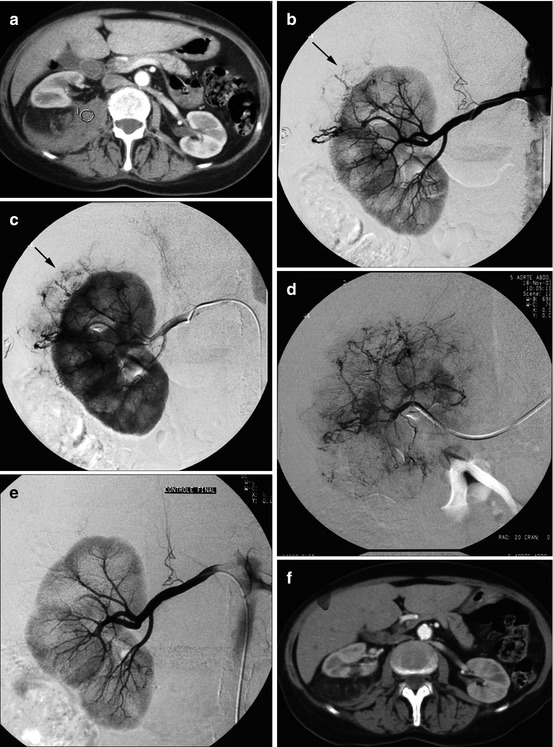
Fig. 13.9
Severe back pain and hemodynamic collapsus in a 57-year-old W. (a) Large kidney, associated with an important hematoma (hematoma HU densities in the circle) in the surrounding fat, suggesting a ruptured angiomyolipoma. (b, c) Selective catheterization of the right renal artery: normal intrarenal vascularization; abnormal vessels providing blood supply to an angiomyolipoma developed from the external face of the upper pole (arrow) (arteriographic phase: b, then nephrographic phase: c). (d) Selective catheterization of the vascular pedicle feeding the angiomyolipoma. (e) Selective arteriographic control after tumoral devascularization. (f) CT scan 1 month later: resorption of perirenal hematoma
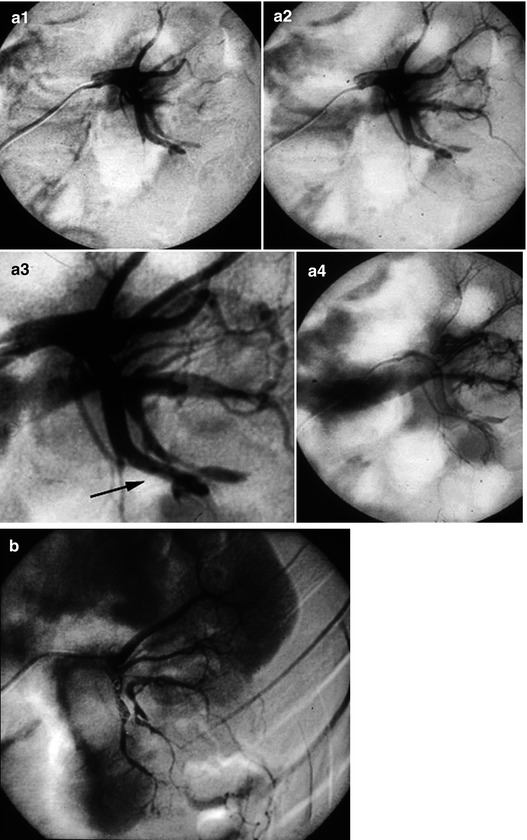
Fig. 13.10




Arteriography performed 4 h after renal biopsy because of the association of back pain, collapsus, and perirenal hematoma on ultrasound. (a1–a4) Selective injection of the artery feeding the left kidney that was biopsied: a massive venous return was noted from the first second, corresponding to a traumatic arteriovenous fistula in the lower part of the sinus (arrow). (b) Control after distal occlusion by coils of the artery supplying the fistula
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


