We hypothesized that uptitration of β blockade and adjustment of pacing parameters to achieve a prevalence of single chamber atrial inhibited rate-responsive (AAIR) pacing in patients with dual-chamber implantable cardioverter–defibrillators (ICDs) would result in maximization of β-blocker dosage and thus decrease appropriate ICD therapies. We included patients with ischemic or dilated cardiomyopathy and implanted ICDs without contraindications to β blockers and atrioventricular conduction disturbances. Two 6-month periods were compared: clinically guided phase (pacing function set at back-up dual-chamber rate-responsive pacing mode at a lower rate of about 40 beats/min) and pacing-guided phase, during which β-blocker dosage was titrated with a target of achieving >90% AAIR pacing (lower rate 60 beats/min). Sixty-one patients (64.2 ± 8.3 years old) were included. During the pacing-guided phase the target of ≥90% AAIR pacing was achieved in 80.3% of patients. Mean metoprolol dose during the clinically guided phase was 96.7 ± 29.4 versus 127.0 ± 39.6 mg/day in the pacing-guided phase (p <0.001). Appropriate ICD therapies were recorded in 35 patients (57.4%) during the clinically guided phase versus 20 (32.8%) during the pacing-guided phase (p <0.001; 1.15 and 0.48 appropriate ICD therapies per patient, respectively, p <0.001). In multivariate analysis, AAIR pacing and β-blocker dose were inversely related to appropriate ICD therapies. In conclusion, a pacing-guided approach for maximizing β-blocker doses guided by maximizing AAIR pacing in patients with ICDs may be beneficial compared to the conventional strategy. This pacing-guided approach led to higher daily β-blocker doses, which were correlated to fewer appropriate ICD therapies.
The advent of implantable cardioverter–defibrillators (ICDs) has been followed by a gradual expansion of their indications beyond their initial application only for secondary prevention of sudden cardiac death driven by studies showing that ICDs improve survival, being superior to antiarrhythmic drugs for the prevention of sudden cardiac death. Beta-blocking agents, in contrast, constitute an indispensable part of treatment in almost all patients receiving an ICD and have proved effective in decreasing arrhythmias and overall mortality in patients with impaired left ventricular function in general and in patients with ICDs in particular. Implantation of an ICD in a nonpacemaker-dependent patient often results in significant periods of pacing when maximal β-blocking treatment is prescribed, especially when physicians try to attain high, clinical trial level dosages, which often are not reached in everyday practice. Because right ventricular pacing has been shown to be an independent predictor for ventricular tachycardia/ventricular fibrillation (VT/VF) occurrence in patients with ICDs, we hypothesized that titration of β blockade and adjustment of pacing parameters to achieve a prevalence of single chamber atrial inhibited rate-responsive (AAIR) pacing over indigenous rhythm or dual-chamber rate-responsive (DDDR)/single chamber ventricular inhibited rate-responsive (VVIR) pacing in patients with dual-chamber ICDs would result in maximization of β-blocker doses and potentially decrease appropriate ICD therapies.
Methods
In this single-center crossover cohort study, we included patients with implanted ICDs and the following inclusion criteria: (1) sinus rhythm, (2) ischemic or dilated cardiomyopathy, (3) implantation of dual-chamber ICD within 2 years before enrollment for a class I or IIa indication as indicated by 2008 American College of Cardiology/American Heart Association guidelines for device-based therapy (this criterion was retrospectively assessed because some patients had received the device before publication of these guidelines), (4) ≥2 appropriate ICD therapies within the previous 6 months before enrollment (or ≥1 electrocardiographically documented episode of sustained ventricular arrhythmia within previous 2 months of preimplantation period if the ICD was implanted <6 months before enrollment), (5) absence of contraindications to β-blocking agents leading to discontinuation of these agents, (6) absence of atrioventricular conduction disturbances, (7) life expectancy ≥1 year, and (8) willingness to provide informed consent. All patients underwent implantation with the device in our center and were regularly followed at our arrhythmia outpatient clinic. The study was approved by the competent institutional scientific committee.
This was a crossover study, i.e., all patients submitted to 1 device programming protocol for 6 months and then changed to the other programming protocol for another 6-month period ( Figure 1 ). Thus 2 6-month periods were compared: clinically guided phase (months 1 to 6) when patient treatment was managed by the attending physician with no access to details of pacing mode analysis (treatment was adjusted by clinical parameters and occurrence of appropriate ICD therapies, which constituted the only ICD-related information provided to the attending physician), and the pacing-guided phase (months 7 to 12) when β-blocker dosage was titrated based on device interrogation data to achieve >90% AAIR pacing. A 1-month lead-in period (month 0) preceded the clinically guided period to ensure adequate clinical titration of metoprolol and optimization of medication dosages from the beginning of the clinically guided phase ( Figure 1 ).
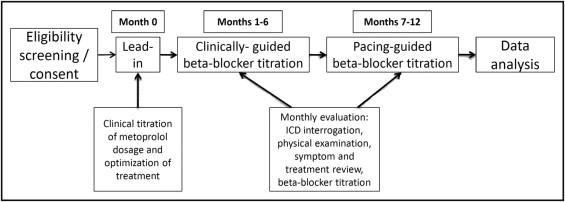
Implanted devices included dual-chamber ICD models with pacing functions from 2 manufacturers (Medtronic, Inc., Minneapolis, Minnesota; Sorin Group, Milan, Italy). At the beginning of the clinically guided phase, ICDs were programmed with a back-up DDDR function with a lower rate limit of about 40 beats/min as recommended by existing guidelines. At the beginning of the pacing-guided phase, pacing mode was programmed at automatic switch mode (between AAIR and DDDR) with AAIR (at a lower rate limit of 60 beats/min) as the primary pacing mode, to respect atrioventricular synchrony and decrease ventricular pacing, with back-up DDDR pacing (for safety reasons in case sudden atrioventricular block ensued). Pacing mode proportions were calculated as percent total indigenous and paced ventricular beats.
Patients were followed monthly over the course of the 1-year follow-up. Appropriateness of ICD therapies was decided by 2 independent electrophysiologists based on recorded electrograms and who were blinded to data of each patient. Blood was drawn at baseline and on a 2-month basis thereafter to perform basic hematologic and biochemical analyses and measurement of plasma N-terminal pro–B-type natriuretic peptide levels. Antiarrhythmics such as sotalol or amiodarone were continued if received on enrollment, but no dosage changes were allowed during follow-up. If an antiarrhythmic agent had to be discontinued during follow-up, the patient was excluded from analysis.
For homogeneity purposes, only 1 β-blocking agent, metoprolol, was used. During the clinically guided phase, metoprolol dosages were titrated according to common clinical practice by the attending physician, with a target heart rate at rest of 55 to 65 beats/min. During the pacing-guided phase, dosage was gradually increased to achieve ≥90% AAIR pacing. If lower AAIR pacing percentages were found, the β-blocker dose was increased; if >5% DDDR pacing was detected, total atrioventricular delay was increased to decrease the proportion of ventricular pacing. The main limiting factors for uptitration of the β blocker were patient-reported symptoms (mainly easy fatigability, dizziness or presyncopal symptoms, decreased exercise tolerance), clinical data (e.g., rales on auscultation), and home and office measurements of arterial pressure (generally avoiding systolic blood pressure <95 mm Hg and diastolic blood pressure <60 mm Hg).
Continuous variables were summarized as mean ± SD and compared using t test. Adequate fit to normal distribution was tested with the Kolmogorov–Smirnov test. For significant deviation from the normal distribution, nonparametric methods were applied (Mann–Whitney U test for independent samples and Wilcoxon test for paired samples). Correlations between continuous variables were tested using Spearman nonparametric test. Categorical variables were summarized as counts and percentages and compared using chi-square test. PASW Statistics 18 (SPSS, Inc., Chicago, Illinois) was used. A p value <0.05 was considered statistically significant.
Results
The records of our outpatient arrhythmia clinic were screened for eligible patients and 65 patients consented to enrollment. Four patients were excluded from the final analysis (3 had to discontinue amiodarone during the study duration and 1 failed to adhere to scheduled follow-up programmed visits). As a result, the analyzable population included 61 patients (64.2 ± 8.3 years of age, 44 men). Patient characteristics are presented in Table 1 . All patients had received dual-chamber (passively fixed atrial lead and right ventricular lead actively fixed at the right ventricular apex) ICDs. During the lead-in period, no patient was hospitalized for heart failure-related events. Most patients had increased N-terminal pro–B-type natriuretic peptide values at baseline (mean 847.1 ± 450.5 pg/ml).
| Parameter | |
|---|---|
| Age (years) | 64.2 ± 8.3 |
| Men | 44 (72%) |
| Underlying structural heart disease | |
| Ischemic cardiomyopathy | 42 (69%) |
| Dilated cardiomyopathy | 19 (31%) |
| New York Heart Association class at baseline | |
| I | 8 (13%) |
| II | 28 (46%) |
| III | 25 (41%) |
| IV | 0 (0%) |
| Left ventricular ejection fraction (%) | 29.4 ± 9.6 |
| Medication at baseline | |
| β Blocker | 61 (100%) |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 58 (95%) |
| Statin | 39 (64%) |
| Diuretic | 48 (79%) |
| Sotalol | 10 (16%) |
| Amiodarone | 15 (25%) |
| N-terminal pro–B-type natriuretic peptide (pg/ml) at baseline | 855.0 ± 450.3 |
| History of appropriate implantable cardioverter–defibrillator therapies before enrollment | 55 (90%) |
| Lower ventricular tachycardia detection zone (beats/min) | 167 ± 5 |
During the clinically guided phase, mean heart rate achieved was 59.8 ± 2.5 beats/min, well within the target range of 55 to 65 beats/min. Mean proportions of indigenous rhythm/pacing modes during this phase were 91.8 ± 7.7% indigenous rhythm, 8.2 ± 7.7% DDDR pacing, and 0% AAIR pacing (during this phase pacing was programmed at a back-up DDDR mode with a lower rate limit of about 40 beats/min). Proportions were, as expected, completely different during the pacing-guided phase (6.5 ± 2.7% indigenous rhythm, 91.7 ± 4.3% AAIR pacing, 1.8 ± 2.1% DDDR pacing). The target of >90% AAIR pacing during the pacing-guided phase was achieved in 49 of 61 patients (80.3%). Ventricular pacing (DDDR) was low with either strategy but significantly lower during the pacing-guided phase (8.2 ± 7.7% vs 1.8 ± 2.1%, respectively, p <0.001, Wilcoxon sign-rank test).
Mean dose of metoprolol attained during the clinically guided phase was 96.7 ± 29.4 versus 127.0 ± 39.6 mg/day in the pacing-guided phase (p <0.001, Wilcoxon sign-rank test). During the clinically guided phase, the ICD was appropriately activated in 35 patients (57.4%) versus 20 (32.8%) during the pacing-guided phase (p <0.001). Counts of appropriate ICD therapies per patient were 1.15 in the clinically guided phase versus 0.48 in the pacing-guided phase (p <0.001, Wilcoxon sign-rank test; Figure 2 ). Table 2 presents univariate correlations between treatment and pacing parameters. It is evident that in the 2 phases metoprolol daily dose was inversely correlated to ICD therapies.
| Spearman Correlation Coefficient | p Value | |
|---|---|---|
| Clinically guided phase | ||
| Number of implantable cardioverter–defibrillator therapies | ||
| Metoprolol dose | −0.596 | <0.001 |
| Percent ventricular pacing | 0.386 | 0.002 |
| Percent indigenous rhythm | −0.385 | 0.002 |
| Metoprolol dose | ||
| Percent ventricular pacing | −0.221 | 0.087 |
| Pacing-guided phase | ||
| Number of implantable cardioverter–defibrillator therapies | ||
| Metoprolol dose | −0.679 | <0.001 |
| Percent ventricular pacing | 0.754 | <0.001 |
| Percent indigenous rhythm | 0.513 | <0.001 |
| Percent single chamber atrial inhibited rate-responsive pacing | −0.706 | <0.001 |
| Metoprolol dose | ||
| Percent ventricular pacing | −0.483 | <0.001 |
| Percent single chamber atrial inhibited rate-responsive pacing | 0.504 | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


