Both urine and serum neutrophil gelatinase-associated lipocalin (NGAL) reflect active chronic kidney disease and predict acute kidney injury. However, a direct comparison of these markers in acute decompensated heart failure has not been performed. We prospectively evaluated 93 patients admitted with acute decompensated heart failure and treated with intravenous furosemide and measured both systemic (serum) and urine NGAL levels and their corresponding markers of estimated glomerular filtration rate, natriuresis (urine sodium), and diuretic response (net output, urine sodium/furosemide ratio). In our study cohort, the median urine and serum NGAL level was 34 ng/ml (interquartile range 24 to 86) and 252 ng/ml (interquartile range 175 to 350), respectively. The urine and serum NGAL levels correlated modestly (r = 0.37, p <0.001). Higher urine (but not systemic) NGAL correlated with the markers of impaired natriuresis and reduced diuresis (p <0.005 for all). In contrast, higher serum NGAL demonstrated a stronger relation with reduced glomerular filtration function (p <0.0001). Both markers predicted acute kidney injury (urine NGAL, odds ratio 1.7, p = 0.035; serum NGAL, odds ratio 1.9, p = 0.009). In conclusion, in patients with acute decompensated heart failure, urine NGAL levels reflect renal distal tubular injury with impaired natriuresis and diuresis, and systemic NGAL levels demonstrate a stronger association with glomerular filtration function. Both systemic and urine NGAL predict worsening renal function.
The inflammatory and oxidative stress responses accompanying renal injury have long been investigated as determinants that drive renal sodium retention, independent of the glomerular filtration rate (GFR). Tubulointerstitial inflammation and oxidative stress enhance local angiotensin II generation, induce proximal tubule sodium reabsorption, compromise dopamine D1 receptor and nitric oxide-mediated sodium excretion, and upregulate distal tubule sodium reabsorption in both the thick ascending limb and the collecting ducts. In acute decompensated heart failure (ADHF), in which worsening renal function limits effective diuresis to relieve the volume overload, such mechanisms might contribute to impaired natriuresis and diuretic resistance. No study has assessed the markers of renal tubular inflammatory and oxidative stress such as neutrophil gelatinase-associated lipocalin (NGAL) with the clinical measures of natriuresis and diuresis in the heart failure setting. In a cohort of patients admitted with ADHF and treated with intravenous diuretics, we examined the relation of systemic and urine NGAL, as the markers of renal inflammatory and oxidative stress, with the clinical measures of renal function, including GFR, natriuresis, and diuresis, and clinical outcomes.
Methods
This was a single-center, prospective study cohort of 93 patients admitted with ADHF. The Cleveland Clinic institutional review board approved the present study, and all subjects gave informed consent. The inclusion criteria were as follows: age ≥18 years, an admission diagnosis of ADHF, and the requirement for intravenous furosemide therapy for fluid retention. The exclusion criteria included previous abdominal or thoracic surgery within the previous 3 months, anticipated discharge from the hospital within 24 hours, urinary tract infection or bacteremia, renal replacement therapy or anuria, and an inability to provide informed consent or comply with the study protocol. The net fluid output and weight loss were recorded for ≤5 days after the baseline NGAL measurements or until discharge.
Simultaneous systemic (serum) and urine samples were collected at baseline after initiation of diuretic therapy, processed, separated into aliquots, and immediately frozen at −80°C until analyzed. The net fluid output and weight loss were then followed for ≤5 days after the baseline NGAL measurements or until discharge. All laboratory analyses were performed with investigators unaware of the cardiorenal indexes and clinical outcomes data. The serum and urine NGAL levels were measured by an enzyme-linked immunosorbent assay (catalog no. KIT 036, BioPorto Diagnostics, Gentofte, Denmark). The minimum detection limit of the assay was 4 pg/ml. The intra-assay and interassay coefficient of variation was 1.1% and 3.2%, respectively, at 65 ng/ml.
Urine sodium (uNa) was measured using ion selective electrode, and urine creatinine was measured by the Roche enzymatic assay within the Cleveland Clinic Reference Laboratory. The intra-assay and interassay coefficient of variation for uNa was 0.3% and 0.6%, respectively. The intra-assay and interassay coefficient of variation for urine creatinine was 0.9% and 2.1%, respectively. Urine furosemide was assessed by NMS Labs (Willow Grove, Pennsylvania) using high-performance liquid chromatography. The minimum detection limit of the assay was 1.0 μg/ml, and the intra-assay and interassay coefficient of variation was 9.5% and 7.3%. In this study, the uNa/urine furosemide ratio represents the estimated natriuretic effect from diuretic therapy. Complete blood counts were collected at the Cleveland Clinic Reference Laboratory using a Sysmex XE-2100 automated hematology analyzer (Sysmex America, Mundelein, Illinois) as a part of standard care. Baseline complete blood counts were collected on the same day as the baseline NGAL measurements. If the baseline complete blood counts were not available, complete blood counts were obtained from the closest date within 90 days of the baseline NGAL measurements. Serum creatinine and blood urea nitrogen levels were monitored for ≤5 days after the baseline NGAL measurements or until discharge. The estimated GFR was calculated using the 4-variable Modification of Diet in Renal Disease study equation. Acute kidney injury (AKI) was defined as a serum creatinine increase of ≥0.3 mg/dl, chosen as a simplified version of the Risk, Injury, Failure, Loss, and End-stage kidney disease and Acute Kidney Injury Network criteria and commonly used in studies of acute cardiorenal syndrome.
Continuous variables are summarized as the mean ± SD if normally distributed and as the median and interquartile range if non-normally distributed. Systemic and urine NGAL were non-normally distributed. Normality was assessed using the Shapiro-Wilk W test. Spearman’s rank correlation method was used as a nonparametric measure of association for correlations between systemic and urine NGAL levels and cardiorenal indexes, including natriuretic and diuretic markers, GFR markers, indexes of anemia, and echocardiographic indexes. The Wilcoxon rank-sum or Kruskal-Wallis tests were used to compare the differences in systemic or urine NGAL across clinical categories, including gender, race, ischemic etiology, a history of hypertension, diabetes mellitus, chronic kidney disease, medication use, and the presence of anemia. Differences in the clinical variables, including natriuretic and diuretic markers, across the median systemic and urine NGAL levels were assessed using either the Wilcoxon rank-sum test for non-normally distributed variables or the Student t test for normally distributed variables. Differences in proportions were assessed using contingency table analysis. Odds ratios for the development of AKI within 5 days of baseline were calculated using logistic regression analysis and evaluated according to the likelihood ratio test. Predictor variables in our logistic regression analyses included standardized natural logarithm transformed serum NGAL, standardized natural-logarithm transformed urine NGAL, serum NGAL ≥250 ng/ml, urine NGAL ≥64 ng/ml, standardized estimated GFR, and standardized serum creatinine. A serum NGAL level of 250 ng/ml and urine NGAL level of 64 ng/ml were the optimal cutpoints maximizing the sensitivity and specificity for the prediction of AKI in the receiver operating characteristic curve analysis. All p values reported are from 2-sided tests, and p <0.05 was considered statistically significant. Statistical analyses were performed using JMP, version 9.0.0 (SAS Institute, Cary, North Carolina).
Results
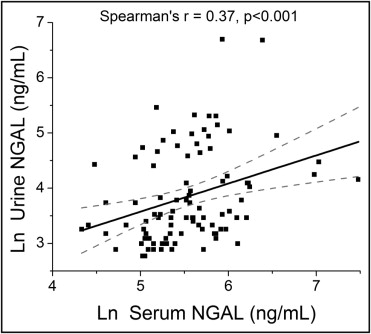
The baseline characteristics of our study cohort stratified by GFR are listed in Table 1 . The mean and median serum NGAL level was 300 ± 230 ng/ml and 252 ng/ml (interquartile range 175 to 350), respectively, and the mean and median urine NGAL level was 75 ± 120 ng/ml and 34 ng/ml (interquartile range 24 to 86), respectively. The serum and urine NGAL levels only correlated modestly (r = 0.37, p <0.001; Figure 1 ).
| Variable | Overall Cohort | GFR (ml/min/1.73 m 2 ) | p Value | |
|---|---|---|---|---|
| <60 | ≥60 | |||
| Age (years) | 64 ± 14 | 68 ± 14 | 58 ± 13 | <0.001 |
| Men | 62 (67%) | 31 (57%) | 31 (79%) | 0.023 |
| Body mass index (kg/m 2 ) | 33 ± 9 | 33 ± 8 | 33 ± 10 | 0.588 |
| Black | 28 (30%) | 15 (28%) | 13 (33%) | 0.565 |
| White | 63 (68%) | 37 (69%) | 26 (67%) | 0.851 |
| Ischemic heart failure etiology | 34 (40%) | 20 (41%) | 14 (38%) | 0.780 |
| Hypertension | 61 (66%) | 40 (74%) | 21 (54%) | 0.043 |
| Diabetes mellitus | 40 (43%) | 26 (48%) | 14 (36%) | 0.237 |
| Anemia | 54 (58%) | 35 (65%) | 19 (49%) | 0.121 |
| Echocardiographic indexes | ||||
| Left ventricular mass index (g/m 2 ) | 177 ± 60 | 171 ± 58 | 185 ± 63 | 0.327 |
| Left ventricular end-diastolic volume index (ml/m 2 ) | 90 ± 47 | 85 ± 49 | 96 ± 43 | 0.118 |
| Left ventricular ejection fraction (%) | 34 ± 17 | 37 ± 17 | 29 ± 16 | 0.037 |
| Diastolic stage III | 60 (72%) | 32 (67%) | 28 (80%) | 0.175 |
| Medications | ||||
| Angiotensin-converting enzyme inhibitors and/or angiotensin receptor blockers | 40 (46%) | 20 (39%) | 20 (56%) | 0.132 |
| β Blockers | 57 (66%) | 33 (65%) | 24 (67%) | 0.850 |
| Spironolactone | 26 (30%) | 16 (31%) | 10 (28%) | 0.718 |
| Loop diuretics | 72 (83%) | 43 (84%) | 29 (81%) | 0.649 |
| Digoxin | 20 (23%) | 10 (20%) | 10 (28%) | 0.375 |
| Laboratory data | ||||
| Serum creatinine (mg/dl) | 1.6 ± 0.9 | 2.1 ± 0.9 | 1.0 ± 0.2 | <0.0001 |
| Blood urea nitrogen (mg/dl) | 39 ± 22 | 51 ± 22 | 22 ± 8 | <0.0001 |
| Brain type natriuretic peptide (pg/ml) | 0.667 | |||
| Median | 897 | 901 | 805 | |
| Interquartile range | 458–1,949 | 467–1,899 | 427–2,004 | |
| Intravenous furosemide dose (mg/day) | 225 ± 155 | 255 ± 158 | 182 ± 142 | 0.019 |
| Systemic neutrophil gelatinase-associated lipocalin (ng/ml) | <0.0001 | |||
| Median | 252 | 306 | 172 | |
| Interquartile range | 175–350 | 252–408 | 150–210 | |
| Urine neutrophil gelatinase-associated lipocalin (ng/ml) | 0.009 | |||
| Median | 34 | 44 | 28 | |
| Interquartile range | 24–86 | 28–114 | 22–52 | |
Higher systemic (but not urine) NGAL levels were associated with advanced age (r = 0.21, p = 0.044). The systemic and urine NGAL levels did not correlate with the systemic brain natriuretic peptide levels and did not differ according to gender, race, ischemic etiology, history of hypertension, or diabetes mellitus (p >0.10 for all). However, patients with greater than median urine NGAL levels did have a greater prevalence of hypertension or diabetes mellitus. Systemic NGAL levels were lower in patients taking angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (median 225 ng/ml, interquartile range 166 to 292, vs median ng/ml, 302, interquartile range 185 to 401; p = 0.013), but systemic or urine NGAL did not differ according to other medication use (p >0.33 for all).
Patients with greater than the median systemic NGAL levels demonstrated a greater prevalence of diastolic stage III and lower left ventricular end-diastolic volume index. In addition, both greater than the median systemic and urine NGAL levels were associated with a greater left ventricular ejection fraction ( Table 1 ). Systemic and urine NGAL levels both correlated with indexes of anemia, including red blood cell count (systemic NGAL, r = −0.31, p = 0.003; urine NGAL, r = −0.26, p = 0.012), hemoglobin (systemic NGAL, r = −0.35, p <0.001; urine NGAL, r = −0.28, p = 0.007), hematocrit (systemic NGAL, r = −0.37, p <0.001; urine NGAL, r = −0.27, p = 0.008), and red blood cell distribution width (systemic NGAL, r = 0.25, p = 0.018). With anemia defined as hemoglobin <12 g/dl if male and <11 g/dl if female, the prevalence of anemia in our cohort was 58% (n = 54). Greater systemic NGAL levels were associated with the presence of anemia (odds ratio [OR] 1.87, 95% confidence interval [CI] 1.17 to 3.20; p = 0.008), but greater urine NGAL levels was not (p = 0.11).
High systemic NGAL levels demonstrated relatively strong associations with markers of poor glomerular filtration function (including the GFR, serum creatinine, and blood urea nitrogen; Table 2 ). In contrast, urine NGAL was modestly associated with GFR and serum creatinine and was not associated with blood urea nitrogen ( Table 2 ). In addition, the serum NGAL levels were greater in patients with a baseline serum creatinine level of ≥1.4 mg/dl (median 330, interquartile range 258.5 to 447 vs median 186, interquartile range 152.5 to 253, p <0.0001), and urine NGAL levels were not (p = 0.31). In addition, the systemic NGAL levels were greater in subjects with a history of chronic kidney disease (median 340 ng/ml, interquartile range 253 to 445 vs median 216 ng/ml, interquartile range 168 to 303, p = 0.003), but the urine NGAL levels were not (median 44 ng/ml, interquartile range 31 to 85 vs median 32, interquartile range 24 to 98, p = 0.227).
| Variable | Systemic NGAL (ng/ml) | Urine NGAL (ng/ml) | ||
|---|---|---|---|---|
| Spearman r | p Value | Spearman r | p Value | |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | −0.69 | <0.0001 | −0.24 | 0.021 |
| Serum creatinine (mg/dl) | 0.68 | <0.0001 | 0.21 | 0.043 |
| Blood urea nitrogen (mg/dl) | 0.66 | <0.0001 | 0.15 | 0.148 |
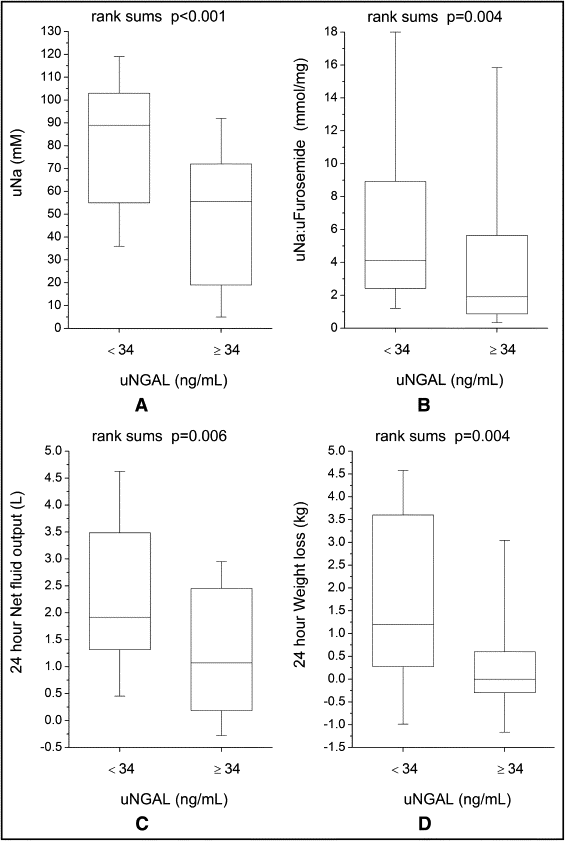
Compared to those with lower (less than the median) urine NGAL levels, the patients with higher (greater than the median) urine NGAL levels demonstrated a reduced natriuretic response (including lower spot uNA [median 56 mM, interquartile range 18 to 73, vs median 89, interquartile range 53 to 105 mM, p <0.001]; lower urine sodium/furosemide ratio [median 1.9 mmol/mg, interquartile range 0.8 to 6.5 vs median 4.1 mmol/mg, interquartile range 2.4 to 9.6, p = 0.004]; lower fractional excretion of sodium [median 1.0%-units, interquartile range 0.3 to 2.9 vs median 3.0%-units, interquartile range 1.2 to 7.3, p = 0.002]; lower fractional exertion of sodium/urine furosemide ratio [median 0.04%-units × L/mg, interquartile range 0.01 to 0.23 vs median 0.18%-units × L/mg, interquartile range 0.05 to 0.82, p = 0.006]) and reduced diuresis during the 24-hour period after the baseline NGAL measurements (including lower 24-hour diuresis [net fluid output 1.3 ± 1.4 L vs 2.3 ± 2.0 L, p = 0.006] and less 24-hour weight loss [0.4 ± 1.6 kg vs 1.8 ± 2.6 kg, p = 0.004]; Figure 2 and Table 3 ). Urine NGAL remained associated with the natriuretic and diuretic markers after adjustment for baseline GFR on multivariate linear regression analysis and in the subgroup analysis after stratification by GFR of 60 ml/min/1.73 m 2 . Urine NGAL also remained associated with the natriuretic and diuretic markers in heart failure with preserved versus reduced ejection fraction (left ventricular ejection fraction ≥45% vs <45%) subgroups. In contrast, the markers of impaired natriuresis and reduced 24-hour diuresis did not differ across the median serum NGAL levels (p >0.07 for all) and serum NGAL did not correlate with markers of impaired natriuresis and 24-hour diuresis ( Table 3 ).