Evaluation of hypertrophic cardiomyopathy (HC) in young patients is limited by lack of age-specific norms for wall thickness on cardiovascular magnetic resonance (CMR) images. Left ventricular strain may have a role in identifying and risk stratifying patients with HC, but few data exist for strain measurement on CMR images. In 30 patients (14.1 ± 3.2 years) with clinically diagnosed HC and 24 controls (15.6 ± 2.8 years), strain (radial, longitudinal, and circumferential) was evaluated by 2 experienced readers using CMR feature tracking. In patients with HC, hypertrophied segments had decreased radial (28.0 ± 5.2% vs 58.6 ± 3.9%, p = 0.0002), circumferential (−23.7 ± 1.1% vs −28.3 ± 0.8%, p = 0.004), and longitudinal (−11.2 ± 1.2% vs −21.7 ± 0.8%, p <0.0001) strains versus control segments. Hypertrophied segments had decreased longitudinal (basal segments −12.2 ± 1.9% vs −22.6 ± 1.2%, p = 0.0002), radial (basal segments 22.7 ± 10.8% vs 78.8 ± 7.2%, p = 0.0001), and circumferential (basal segments −22.4 ± 1.7% vs −30.6 ± 1%, p = 0.0004) strains versus nonhypertrophied segments in patients with HC. Longitudinal strain had the lowest intraobserver and interobserver variabilities (coefficient of variability −15.7% and −18.5%). After a median follow-up of 28.1 months (interquartile range [IQR] 4.2 to 33.1), 7 patients with HC with an adverse event outcome (5 ventricular tachycardia, 1 appropriate implantable cardioverter-defibrillator discharge, and 1 death) had reduced global radial (median 39.7%, IQR 39.6% to 46.6% vs 65.4%, IQR 46.1% to 83.4%, p = 0.01) and longitudinal strains (median −16.5%, IQR −18.7% to −15.5% vs −19.7%, IQR −23.8% to −17.5%, p = 0.046) compared with patients with HC without an event. In conclusion, CMR feature tracking detects differences in global and segmental strains and may represent a novel method to predict clinical outcome in patients with HC. Further study is necessary to evaluate longitudinal changes in this population.
Highlights
- •
In young patients with hypertrophic cardiomyopathy, strain by feature tracking is reduced in hypertrophied segments.
- •
Global radial and longitudinal strains are decreased in patients with hypertrophic cardiomyopathy with adverse events.
- •
Intraobserver and interobserver variabilities are high, necessitating refinement before clinical use.
Hypertrophic cardiomyopathy (HC) is characterized by myocardial disarray, hypertrophy, and fibrosis and is associated with significant morbidity and mortality. Cardiovascular magnetic resonance (CMR) imaging is a standard tool for diagnosis and evaluation of HC, but age-specific normative data in the pediatric population, particularly for wall thickness, are limited. In the absence of late gadolinium enhancement, quantitative evaluation in this population may be limited. Strain, a measure of myocardial deformation, is an increasingly useful assessment of regional and global cardiac functions, which has been associated with clinical outcomes in several cardiac conditions. In patients with HC, decreased strain by 2-dimensional speckle tracking echocardiography has been associated with areas of hypertrophy and fibrosis and with adverse cardiac events in adults. Previous reports of strain measurement from standard cine CMR images have been limited to adapted application of echocardiographic software. Feature tracking, a method of strain assessment using standard cine CMR images, has been validated against traditional myocardial tagging methods in normal subjects and patients with Duchenne muscular dystrophy ; however, few data exist related to HC or clinical outcome. This study aimed to determine whether left ventricular strain by feature tracking could differentiate pediatric, adolescent, and young adult patients with HC from controls and the potential relation of decreased strain to clinical outcome.
Methods
This single-center retrospective study included all patients ≤21 years with phenotypic or genotypic HC who underwent clinically indicated CMR from 2007 to 2012. HC was defined as positive genetic testing for a mutation known to be associated with HC or clinical characteristics of asymmetric septal hypertrophy, septal hypertrophy with systolic anterior motion of the mitral valve, or total left ventricular mass >2 SDs above the mean for body surface area without identifiable origin. Those with coexisting congenital heart disease, an underlying syndrome, or storage condition predisposing to secondary HC were excluded. Controls included all patients ≤21 years who underwent clinically indicated CMR from 2007 to 2012 with normal left ventricular ejection fraction (LVEF), normal left ventricular mass by CMR and no hypertrophy, no congenital heart disease or genotype associated with congenital heart disease, and no history of ventricular arrhythmia. This study was approved by the institutional review board, and the requirement for informed consent was waived.
Patient charts were reviewed for demographic data, implantable cardioverter-defibrillator placement, and clinical outcomes. Composite adverse outcome was defined as death, sustained or nonsustained ventricular tachycardia, ventricular fibrillation, or appropriate implantable cardioverter-defibrillator discharge. Length of follow-up was defined as the time from CMR to last clinical documentation.
CMR data were obtained using a commercially available 1.5-T magnetic resonance imaging scanner (Achieva or Ingenia; Philips, Best, the Netherlands). Cine images were obtained using a breath-hold, electrocardiographic-gated, segmented k-space, steady-state free-precession sequence, with 30 phases per cardiac cycle. Data were postprocessed using an off-line workstation with QMass MR (Medis, Leiden, the Netherlands). Although normative data exist for indexed left ventricular mass by CMR in the pediatric population, no such data exist for maximal wall thickness. Therefore, qualitatively hypertrophied segments were identified by agreement of 2 experienced readers, blinded to patient outcome, and were mapped on a 16-segment model.
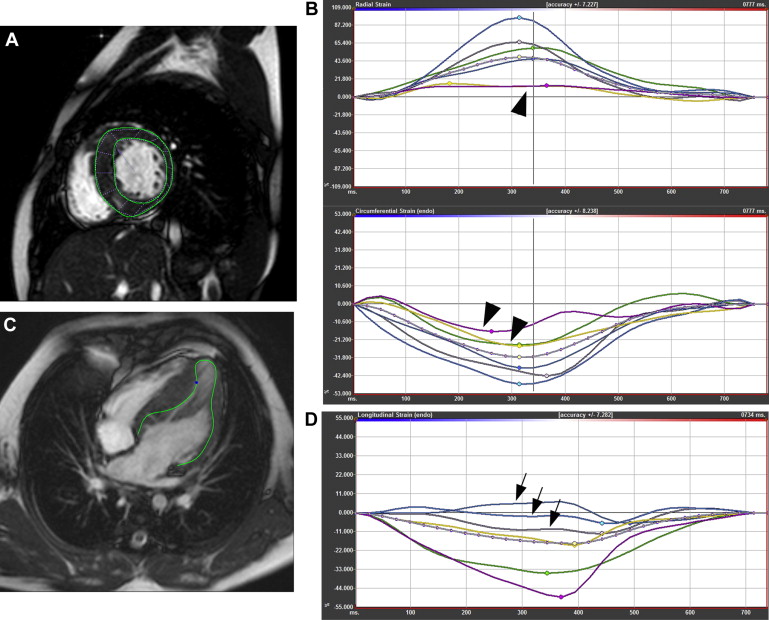
Using a feature-tracking software (TomTec, Unterschleissheim, Germany), 2 separate readers manually drew contours at the endocardial border ( Figure 1 ) that were evaluated for adequate tracking and edited as necessary. Basal, midventricular, and apical short-axis images were analyzed for circumferential and radial strains. Two-, 3-, and 4-chamber images were used for longitudinal strain measurement. Lagrangian strain was measured at the endocardium. Global strain measurements were calculated as the average of the peak values of the 16 segments.
Data are presented as frequency (percentage) for categorical variables and mean ± SD or median (interquartile range), as appropriate, for continuous variables. Increased LVEF is a characteristic of the HC population; we compared strain measurements between patients with HC and controls using analysis of variance controlling for LVEF, to determine whether global or segmental strain could differentiate patients from controls with a similar LVEF. Repeated-measures analysis of variance accounting for correlation across segments within patients was used to compare strain in segments with and without hypertrophy from patients with HC and segments from controls. Strain was also compared in patients with or without adverse clinical outcome, using Wilcoxon rank sum test. Odds ratio (OR) and 95% confidence interval (CI) of having an adverse clinical outcome per percent decrease in strain are reported. Intraobserver and interobserver variabilities of strain are represented as mean difference with limits of agreement and coefficient of variability (COV). All analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, North Carolina), with statistical significance set at a p value <0.05 using a 2-sided test.
Results
Demographic data are listed in Table 1 . Additional demographic data by case with specific mutations were previously published. During the study period, 51 patients were referred for CMR for possible HC, with 30 ultimately included in the study population. Of those excluded, 2 had associated congenital heart disease, 1 had a syndrome associated with secondary HC, and 18 lacked adequate phenotypic or genotypic evidence of HC. In the study population, 12 patients underwent genotype testing, and 11 were positive for known mutations associated with HC. Of the 11 genotype-positive patients, 6 were phenotype negative. Indexed left ventricular mass was greater than the ninety-fifth percentile in 15 patients. Hypertrophy was identified in at least 1 segment in 24 patients (80%), with a median of 5 segments per patient (interquartile range 2 to 6 segments). Left ventricular outflow tract gradient >30 mm Hg by echocardiography was present in 8 patients (27%). This cohort was largely asymptomatic, with no patients in clinical heart failure, 4 patients (13%) in New York Heart Association class II, and the remainder in class I.
| Variable | HC (n = 30) | Controls (n = 24) | p-Value |
|---|---|---|---|
| Age at CMR (years) | 14.1 ± 3.2 | 15.6 ± 2.8 | 0.08 |
| Male sex | 17 (57%) | 13 (54%) | 0.85 |
| Body surface area (m 2 ) | 1.7 ± 0.3 | 1.8 ± 0.3 | 0.11 |
| Left ventricular ejection fraction (%) | 65.4 ± 5.8 | 59.4 ± 3.0 | <0.001 |
| Indexed left ventricular end diastolic volume (ml/m 2 ) | 89 ± 16 | 86.5 ± 13.7 | 0.58 |
| Indexed left ventricular mass (g/m 2 ) | 88 ± 40 | 57 ± 12 | <0.001 |
| CMR maximal wall thickness (mm) | 18.2 ± 8.1 | 7.7 ± 1.8 | <0.001 |
Control patients were referred for evaluation of possible arrhythmogenic right ventricular cardiomyopathy (10), family history of aortic pathology (4), coronary artery anatomy (4), possible left ventricular hypertrophy (3, all patients having normal indexed left ventricular mass by CMR and no focal hypertrophy), chest pain (1), possible intracardiac tumor (1), and suspected aortic arch anomaly (1). No structural or functional abnormalities were detected in this population. LVEF was higher in patients with HC in comparison with controls ( Table 1 ).
Patients with phenotypic HC had decreased global longitudinal strain compared with controls while controlling for LVEF (−18.5 ± 1.0% vs −21.7 ± 0.9%, p = 0.04); however, circumferential (−28.3 ± 0.7% vs −28.0 ± 0.8%, p = 0.83) and radial (58.6 ± 3.2% vs 56.9 ± 3.7%, p = 0.76) strains did not differ. Patients who were genotype positive but phenotype negative had no difference in global circumferential (−27.0 ± 1.4% vs −27.9 ± 0.8%, p = 0.58), radial (48.8 ± 6.8% vs 55.5 ± 3.7%, p = 0.36), or longitudinal (−22.7 ± 1.7% vs −21.7 ± 0.9%, p = 0.57) strain compared with controls.
On a segmental level, segments with hypertrophy had reduced radial and longitudinal strains compared with controls, whereas segments without hypertrophy had greater radial and circumferential strains compared with controls ( Figure 2 ). After controlling for LVEF, segments without hypertrophy no longer differed from controls for radial (28.0 ± 5.2% vs 58.6 ± 3.9%, p = 0.0002), circumferential (−23.7 ± 1.1% vs −28.3 ± 0.8%, p = 0.004), or longitudinal (−11.2 ± 1.2% vs −21.7 ± 0.8%, p <0.0001) strain.
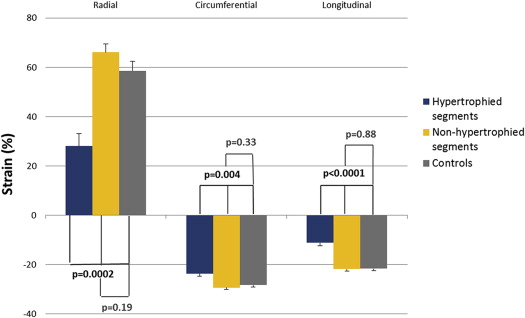
Comparing hypertrophied segments to nonhypertrophied segments in patients with HC, hypertrophied segments had decreased longitudinal (basal segments −12.2 ± 1.9% vs −22.6 ± 1.2%, p = 0.0002; midsegments −11.5 ± 1.5% vs −20.6 ± 0.6%, p <0.0001), radial (basal segments 22.7 ± 10.8% vs 78.8 ± 7.2%, p = 0.0001; midsegments 40.6 ± 6% vs 63.7 ± 4.5%, p = 0.001), and circumferential (basal segments −22.4 ± 1.7% vs −30.6 ± 1%, p = 0.0004) strains. There was no difference in strain at the apical level, although there were few apical segments with hypertrophy.
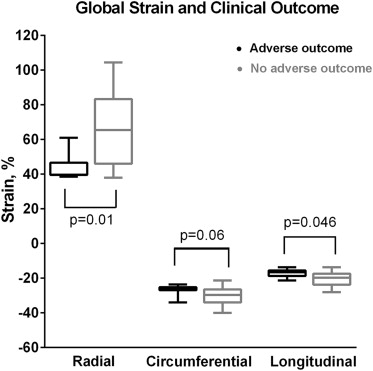
After a median follow-up of 14.2 months (interquartile range 0.8 to 34), 7 patients met the composite adverse outcome: 5 patients had nonsustained ventricular tachycardia, 1 patient had an appropriate implantable cardioverter-defibrillator discharge, and 1 patient died suddenly during sleep. Global radial and longitudinal strains were decreased in those with adverse outcomes compared with patients with HC without an adverse outcome ( Figure 3 ). Excluding the 6 genotype-positive phenotype-negative patients, these differences remained significant. There were increased odds of having an adverse event per percent decrease in global radial strain (OR 1.13, 95% CI 1.00 to 1.27). Odds for an adverse event were not significant for decreased longitudinal or circumferential strain (global longitudinal strain, OR 1.37, 95% CI 0.99 to 1.88 and global circumferential strain OR 1.23, 95% CI 0.96 to 1.58).