We used virtual histology intravascular ultrasound (VH-IVUS) to assess culprit plaque rupture in 172 patients with ST-segment elevation acute myocardial infarction. VH-IVUS-defined thin-capped fibroatheroma (VH-TCFA) had necrotic core (NC) >10% of plaque area, plaque burden >40%, and NC in contact with the lumen for ≥3 image slices. Ruptured plaques were present in 72 patients, 61% of which were located in the proximal 30 mm of a coronary artery. Thirty-five were classified as VH-TCFA and 37 as non-VH-TCFA. Vessel size, lesion length, plaque burden, minimal lumen area, and frequency of positive remodeling were similar in VH-TCFA and non-VH-TCFA. However, the NC areas within the rupture sites of VH-TCFAs were larger compared to non-VH-TCFAs (p = 0.002), while fibrofatty plaque areas were larger in non-VH-TCFAs (p <0.0001). Ruptured plaque cavity size was correlated with distal reference lumen area (r = 0.521, p = 0.00002), minimum lumen area (r = 0.595, p <0.0001), and plaque area (r = 0.267, p = 0.033). Sensitivity and specificity curve analysis showed that a minimum lumen area of 3.5 mm 2 , a distal reference lumen area of 7.5 mm 2 , and a maximum NC area of 35% best predicted plaque rupture. Although VH-TCFA (35 of 72) was the most frequent phenotype of plaque rupture in ST-segment elevation myocardial infarction, plaque rupture also occurred in non-VH-TCFA: pathologic intimal thickening (8 of 72), thick-capped fibroatheroma (1 of 72), and fibrotic (14 of 72) and fibrocalcified (14 of 72) plaque. In conclusion, not all culprit plaque ruptures in patients with ST-segment elevation myocardial infarction occur as a result of TCFA rupture; a prominent fibrofatty plaque, especially in a proximal vessel, may be another form of vulnerable plaque. Further study should identify additional factors causing plaque rupture.
Rupture of a thin-capped fibroatheroma can lead to thrombosis, acute coronary syndromes, and sudden cardiac death. However, autopsy studies have suggested that non-thin-capped fibroatheromas may also be “vulnerable,” and rupture of a thin fibrous cap overlying a lipid core is not the only pathway to a coronary thrombus. Conversely, healing of a ruptured plaque may be a mechanism of stenosis progression without causing an acute event, while sudden progression may indicate a more complex lesion, thrombus, plaque rupture, or erosion in the setting of an acute event. Virtual histology intravascular ultrasound (VH-IVUS) assesses plaque composition and defines atherosclerotic lesion phenotype, including thin-capped fibroatheroma. There are only a few reports of the use of VH-IVUS in acute ST-segment elevation myocardial infarction (STEMI). In this study, we used VH-IVUS to evaluate the phenotype underlying culprit lesion plaque rupture in patients with STEMI who underwent primary percutaneous coronary intervention.
Methods
Overall, 200 consecutive, prospectively studied patients with acute STEMI underwent primary percutaneous coronary intervention with VH-IVUS imaging of the culprit lesion at 15 centers in Korea. Of these, 172 were amenable to analysis. Bifurcation lesions, ostial lesions, vein graft lesions, arteries with previous stent placement, and pre-IVUS debulking or plaque modification procedures were excluded. Standard coronary risk factors were collected, including age, gender, hypertension (medication treated only), diabetes mellitus (including medication-treated and diet-controlled diabetes or fasting blood glucose level >126 mg/dl), hypercholesterolemia (medication treated or measured >240 mg/dl), current smoking (within the past 12 months), and family history of coronary artery disease (myocardial infarction in a first-degree relative aged <60 years). The protocol was approved by each institutional review board; written informed consent was obtained from all patients.
Coronary angiography was performed after 200 μg of intracoronary nitroglycerin and analyzed with an automated edge detection algorithm (AI 1000; GE Medical Systems, Milwaukee, WI) using standard protocols. Minimal lumen diameter, % diameter stenosis, reference vessel diameter, and Thrombolysis In Myocardial Infarction (TIMI) grade were measured before intervention. The culprit lesion was identified by the combination of electrocardiographic findings, left ventricular wall motion abnormalities, and angiographic lesion morphology. Thrombus aspiration was performed before IVUS imaging using an aspiration catheter (Kaneka Corporation, Osaka, Japan) according to operator discretion, but typically for large thrombi. A commercially available VH-IVUS system (Volcano Therapeutics, Rancho Cordova, California) and 20-MHz transducers were used for all IVUS examinations. After intracoronary administration of 200 μg nitroglycerin, the IVUS catheter was advanced 10 mm distal to the target lesion, and imaging was performed retrograde to the aorto-ostial junction using an electrocardiographically gated automatic pullback device.
Grayscale IVUS analysis was performed according to the criteria of the American College of Cardiology clinical expert consensus document on IVUS. A ruptured plaque contained a cavity that communicated with the lumen with an overlying residual fibrous cap fragment. Positive remodeling was a lesion/mean reference external elastic membrane area >1.05.
Two experienced analysts (S.W.K, Y.J.H.) reviewed the VH-IVUS pullback to assess lesion phenotype and make precise measurements by defining the 2 standard VH-IVUS regions of interest: the inner border (lumen, excluding IVUS-detectable thrombus) and outer border (external elastic membrane). VH-IVUS analyses assessed fibrotic tissue, fibrofatty plaque, necrotic core (NC), and dense calcium, and each component was reported in absolute amounts and as percentages of plaque area. The mean plaque area was measured over the entire length of the lesion. Culprit lesion phenotype was classified as pathologic intimal thickening, VH-IVUS-defined thin-capped fibroatheroma (VH-TCFA), thick-capped fibroatheroma, and fibrotic and fibrocalcific plaque.
Statistical analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina). Continuous variables are presented as mean ± SD, and data were normally distributed and compared using Student’s t test. Logistic regression identified variables to predict plaque rupture. Using sensitivity and (1 − specificity) curves, the optimal threshold of vessel area was identified to predict plaque rupture. Receiver-operating characteristic curves were used to compare the accuracy of indicator variables. Multivariate logistic analysis was used to identify the most important factor to plaque rupture. A p value <0.05 was considered statistically significant.
Results
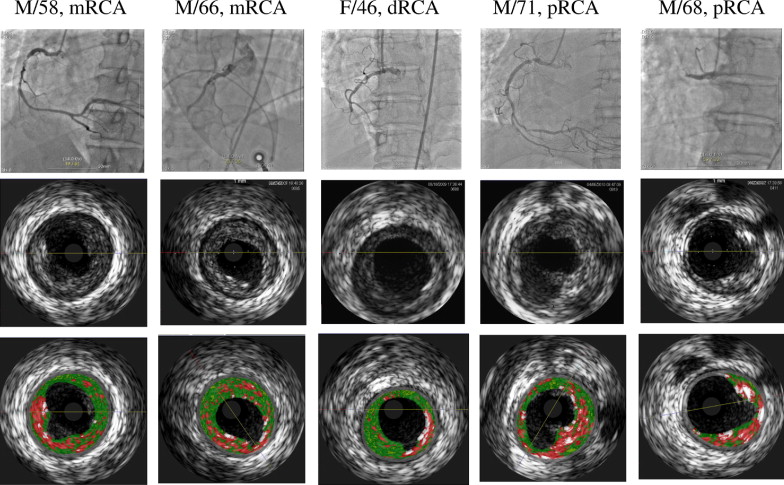
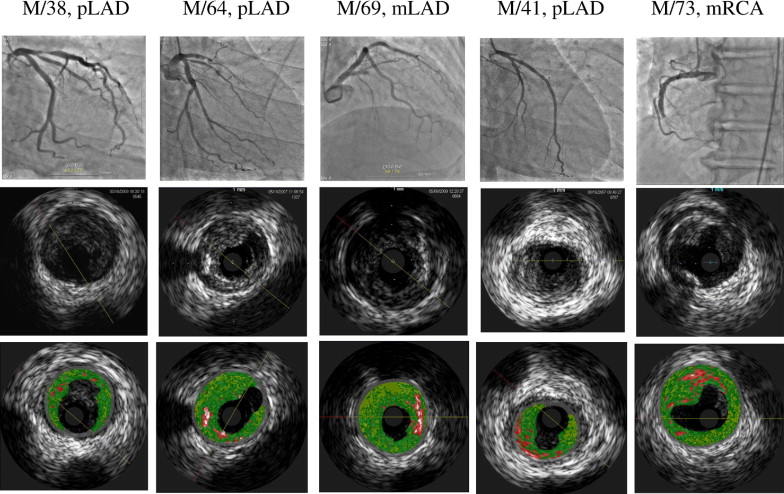
Overall, plaque ruptured was observed in 72 of 172 (41%) patients with acute STEMI ( Figure 1 ) . Of these, 35 had an underlying phenotype of VH-TCFA, and 37 had non-VH-TCFA phenotype. Overall, 61% of ruptured plaques (44 of 72) were located in the proximal 30 mm of a coronary artery. Clinical demographics of overall cohort are listed in Table 1 .
| Variable | VH-TCFA (n = 65) | Non-VH-TCFA (n = 107) | p Value |
|---|---|---|---|
| Age (years) | 60 ± 11 | 58 ± 12 | 0.439 |
| Men | 55 (85%) | 90 (84%) | 1.0 |
| Diabetes mellitus | 15 (23%) | 23 (21%) | 0.838 |
| Hypertension | 28 (43%) | 43 (40%) | 0.731 |
| Current smoker | 31 (48%) | 60 (56%) | 0.491 |
| Hypercholesterolemia ⁎ | 20 (31%) | 23 (22%) | 0.284 |
| Coronary artery treated | |||
| LAD | 24 | 71 | 0.001 |
| LCX | 10 | 12 | |
| RCA | 31 | 24 | |
| Number of narrowed coronary arteries; | 0.255 | ||
| 1 | 27 | 38 | |
| 2 | 17 | 44 | |
| 3 | 21 | 25 | |
| TIMI grade | 0.789 | ||
| 0 | 33 | 45 | |
| 1 | 5 | 6 | |
| 2 | 10 | 26 | |
| 3 | 17 | 30 | |
| Before intervention | |||
| Lesion length (mm) | 20.16 ± 8.89 | 21.78 ± 9.93 | 0.359 |
| Minimal lumen diameter (mm) | 0.54 ± 0.73 | 0.76 ± 0.54 | 0.067 |
| Reference vessel diameter (mm) | 3.32 ± 0.74 | 3.04 ± 0.55 | 0.028 |
| Diameter stenosis (%) | 84.27 ± 19.03 | 75.09 ± 17.66 | 0.009 |
As shown in Table 2 , VH-TCFAs were identified in 37.8% of patients (65 of 172), while 62.2% (107 of 172) were non-VH-TCFAs. Plaque rupture was seen in 53.8% of VH-TCFAs (35 of 65) compared to 34.5% of non-VH-TCFAs (37 of 107) (p = 0.009). Although a VH-TCFA (n = 35) was the most frequent phenotype underlying the 72 plaque ruptures in the 172 patients with STEMI, non-VH-TCFA phenotype included pathologic intimal thickening in 8, thick-cap fibroatheroma in 1, fibrotic plaque in 14, and fibrocalcified plaque in 14. Vessel size, lesion length, plaque burden, minimal lumen area (MLA), and frequency of positive remodeling (57% [20 of 35] vs 56% [21 of 37], p = 0.57) were similar in VH-TCFA and non-VH-TCFA plaque ruptures ( Table 2 ). The sizes of ruptured plaque cavities were similar (p = 0.177), while the MLA of the underlying plaque at the rupture site (excluding the superficial thrombus) was smaller in non-VH-TCFA than in VH-TCFA (p = 0.016). Ruptured plaque cavity size was correlated with distal reference lumen area (r = 0.521, p = 0.00002), MLA (r = 0.595, p <0.0001), and plaque area (r = 0.267, p = 0.033).
| Variable | VH-TCFA (n = 65) | Non-VH-TCFA (n = 107) | p Value |
|---|---|---|---|
| Proximal reference segment | |||
| EEM area (mm 2 ) | 20.5 ± 5.4 | 18.3 ± 6.2 | 0.019 |
| Lumen area (mm 2 ) | 10.8 ± 4.1 | 10.6 ± 4.7 | 0.731 |
| Plaque area (mm 2 ) | 9.7 ± 4.1 | 7.7 ± 3.0 | 0.001 |
| Plaque burden (%) | 46.8 ± 14.0 | 42.1 ± 11.3 | 0.033 |
| Lesion site | |||
| Lesion length (mm) | 22.2 ± 10.0 | 23.7 ± 11.5 | 0.459 |
| Mean EEM area (mm 2 ) | 16.7 ± 5.7 | 15.1 ± 6.1 | 0.094 |
| Mean lumen area (mm 2 ) | 6.4 ± 2.7 | 6.2 ± 2.8 | 0.686 |
| Mean plaque area (mm 2 ) | 10.3 ± 4.0 | 8.9 ± 3.9 | 0.030 |
| Mean plaque burden (%) | 60.5 ± 9.7 | 56.6 ± 10.3 | 0.017 |
| MLA site | |||
| EEM area (mm 2 ) | 17.2 ± 6.7 | 14.5 ± 6.2 | 0.009 |
| Lumen area (mm 2 ) | 3.9 ± 2.2 | 3.8 ± 2.1 | 0.893 |
| Plaque area (mm 2 ) | 13.4 ± 5.9 | 10.6 ± 5.0 | 0.003 |
| Remodeling index | 1.0 ± 0.3 | 0.9 ± 0.2 | 0.328 |
| Maximal NC site | |||
| EEM area (mm 2 ) | 18.9 ± 5.9 | 16.4 ± 6.5 | 0.014 |
| Lumen area (mm 2 ) | 6.6 ± 2.8 | 6.0 ± 3.0 | 0.188 |
| Plaque area (mm 2 ) | 12.3 ± 4.5 | 10.5 ± 4.6 | 0.013 |
| Plaque burden (%) | 64.7 ± 10.9 | 63.2 ± 11.1 | 0.379 |
| Remodeling index | 1.1 ± 0.3 | 1.1 ± 0.2 | 0.402 |
| Distal reference segment | |||
| EEM area (mm 2 ) | 14.0 ± 6.2 | 13.0 ± 6.3 | 0.332 |
| Lumen area (mm 2 ) | 7.8 ± 3.5 | 7.5 ± 3.9 | 0.631 |
| Plaque area (mm 2 ) | 6.2 ± 3.7 | 5.5 ± 3.4 | 0.241 |
| Ruptured VH-TCFA (n = 35) | Ruptured non-VH-TCFA (n = 37) | ||
|---|---|---|---|
| Lesion length (mm) | 18.8 ± 6.9 | 22.2 ± 9.9 | 0.143 |
| Proximal reference segment | |||
| EEM area (mm 2 ) | 21.4 ± 5.6 | 18.8 ± 5.2 | 0.063 |
| Lumen area (mm 2 ) | 11.1 ± 4.1 | 10.1 ± 3.4 | 0.305 |
| Plaque area (mm 2 ) | 10.3 ± 4.2 | 8.72 ± 3.5 | 0.108 |
| Plaque burden (%) | 47.8 ± 12.1 | 45.9 ± 12.7 | 0.540 |
| Ruptured plaque site | |||
| EEM area (mm 2 ) | 19.2 ± 5.3 | 17.5 ± 6.1 | 0.240 |
| Lumen area (mm 2 ) | 7.4 ± 2.7 | 5.9 ± 2.3 | 0.016 |
| Plaque area (mm 2 ) | 11.8 ± 3.8 | 11.7 ± 5.5 | 0.916 |
| Plaque burden (%) | 61.1 ± 10.6 | 64.2 ± 13.4 | 0.295 |
| Remodeling index | 1.1 ± 0.2 | 1.1 ± 0.3 | 0.711 |
| Ruptured cavity (mm 2 ) | 1.1 ± 1.0 | 0.8 ± 0.7 | 0.177 |
| Distal reference segment | |||
| EEM area (mm 2 ) | 15.7 ± 6.8 | 13.8 ± 5.5 | 0.219 |
| Lumen area (mm 2 ) | 8.8 ± 4.3 | 7.7 ± 3.1 | 0.269 |
| Plaque area (mm 2 ) | 6.9 ± 3.7 | 6.1 ± 3.3 | 0.329 |
| Plaque burden (%) | 42.8 ± 13.3 | 42.5 ± 11.8 | 0.936 |
VH-IVUS findings are listed in Table 3 . The % mean NC area (over the length of the lesion) was greater in the VH-TCFA than the non-VH-TCFA group (p = 0.002). Analysis of plaque composition at the rupture site showed larger % NC area in VH-TCFA (p <0.0001), while % fibrofatty area was greater in non-VH-TCFA (p = 0.006).
| Variable | VH-TCFA (n = 65) | Non-VH-TCFA (n = 107) | p Value |
|---|---|---|---|
| Lesion segment | |||
| Mean fibrotic plaque (mm 2 ) | 4.35 ± 2.43 | 3.73 ± 2.40 | 0.111 |
| Mean fibrofatty plaque (mm 2 ) | 0.74 ± 0.63 | 0.71 ± 0.80 | 0.801 |
| Mean NC (mm 2 ) | 1.76 ± 1.19 | 1.00 ± 0.66 | <0.0001 |
| Mean dense calcium (mm 2 ) | 0.71 ± 0.56 | 0.53 ± 0.44 | 0.033 |
| Mean fibrotic plaque (%) | 56.30 ± 11.08 | 59.82 ± 13.07 | 0.067 |
| Mean fibrofatty plaque (%) | 8.85 ± 5.13 | 10.70 ± 6.18 | 0.04 |
| Mean NC (%) | 22.18 ± 7.98 | 16.05 ± 7.24 | <0.0001 |
| Mean dense calcium (%) | 10.83 ± 6.95 | 9.29 ± 6.57 | 0.162 |
| Maximal NC site | |||
| Fibrotic plaque (mm 2 ) | 4.17 ± 2.55 | 3.78 ± 2.51 | 0.339 |
| Fibrofatty plaque (mm 2 ) | 0.37 ± 0.36 | 0.50 ± 0.88 | 0.204 |
| NC (mm 2 ) | 3.73 ± 1.98 | 2.06 ± 1.02 | <0.0001 |
| Dense calcium (mm 2 ) | 1.26 ± 1.03 | 0.99 ± 0.76 | 0.07 |
| Fibrotic plaque (%) | 42.21 ± 12.47 | 49.30 ± 15.85 | 0.002 |
| Fibrofatty plaque (%) | 3.84 ± 3.09 | 5.57 ± 5.16 | 0.019 |
| NC (%) | 39.46 ± 10.04 | 29.81 ± 9.91 | <0.0001 |
| Dense calcium (%) | 14.62 ± 9.60 | 14.74 ± 10.17 | 0.939 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


