Histidine decarboxylase (HDC) is a key determinant of the levels of endogenous histamine that has long been recognized to play important pathophysiological roles during development of chronic heart failure (CHF). Meanwhile, certain genetic variants in HDC gene were reported to affect the function of HDC and associated with histamine-related diseases. However, the relation between polymorphisms of HDC gene and CHF risk remains unclear. This study aims to investigate the associations between 2 nonsynonymous HDC polymorphisms (rs17740607 and rs2073440) and CHF. We designed a 2-stage case–control study, in which we genotyped 439 patients with CHF and 467 healthy controls recruited in Xi’an, China, and replicated this study in 413 patients with CHF and 452 healthy subjects in Kunming, China. We also performed in vitro experiments to further validate the functional consequences of variants positively associated with CHF. The rs17740607 polymorphism showed replicated associations with all-cause CHF according to genotype and allele distribution and also under a dominant and additive genetic model after adjusted for traditional cardiovascular-related factors. Functional experiments further demonstrated that rs17740607 polymorphism decreased the HDC activity. In conclusion, HDC rs17740607 polymorphism is at least a partial loss-of-function variant and acts as a protective factor against CHF, which provides novel highlights for investigating the contribution of CHF.
Chronic heart failure (CHF) is an ever increasing health problem worldwide, and genetic factors are generally believed to contribute greatly to its development. Nevertheless, the genetic architecture of sporadic CHF is poorly characterized yet, and identification of certain novel variants associated with CHF will obviously forward our understanding of personalized prevention and treatment of the disease. Recently, increasing evidence strongly suggested that cardiac endogenous histamine was highly involved in various cardiac dysfunctions and even CHF itself. On the basis of the notion that CHF is the end stage of all kinds of cardiac dysfunctions, factors that affect the endogenous histamine level are probably common hallmarks of CHF despite different causes. Histidine decarboxylase (HDC) is the only rate-limiting enzyme in histamine synthesis and is believed as a determinative factor of the endogenous histamine level. Furthermore, certain polymorphisms in HDC gene were significantly associated with various histamine-related diseases and were presumed to affect the enzyme activity. Therefore, it is reasonable for us to speculate that HDC gene may be also a candidate gene associated with CHF, which, however, has not been investigated in detail so far. We herein performed a 2-stage case–control study in 2 different CHF populations followed by further in vitro functional validation study to investigate the associations between CHF risk and 2 nonsynonymous single-nucleotide polymorphisms (SNPs) in HDC gene.
Methods
Randomly selected northern Han Chinese patients with CHF who were admitted to the First Affiliated Hospital, College of Medicine, Xi’an Jiaotong University, from 2010 to 2014 were enrolled. The primary inclusion criterion is a clinical diagnosis of CHF with abnormal left ventricular function by echocardiography performed on hospital admission and also in accordance with the Framingham criteria. The exclusion criteria were age <18 years and the presence of severe hepatic or renal insufficiency, tumors or malignant disease, acute attack of CHF, or severe acute infection. The cause of CHF was determined in each patient by clinical assessment and echocardiography. The etiology of CHF was classified as ischemic or nonischemic subgroups. Ischemic CHF was defined by at least a 50% narrowing on coronary angiography, a positive stress test, history of an acute coronary syndrome, or previous coronary revascularization. Cases free of these criteria were classified as nonischemic. Patients who could not be clearly classified (n = 34) were excluded from the present study. Finally, a total of 439 patients with CHF were included in the present study. As for the control subjects, 467 unrelated healthy Han persons living in Xi’an with comparable age and gender were included to minimize the bias. The number of cases in the area during the study period determined the sample size. The inclusion criteria were no history of heart failure, normal ventricular function on cardiac imaging, and no evidence of coronary artery disease as determined either by a negative treadmill exercise test or by maximal coronary stenoses of ≤20% on coronary angiography. Exclusion criteria for the controls were personal and family history of cardiovascular disease. A detailed interview addressed to personal and familial history was performed in the framework of a physical examination by expert physicians to identify symptom-free subjects and to exclude those who were suspected of having any form of vascular disease.
To test for independent replication of associations identified in Xi’an population, we recruited additional 413 southern Han Chinese patients with CHF, who were admitted (from 2012 to 2014) to the Kunming General Hospital of Chengdu Military Region and additional 452 health Han controls, who lived in Kunming and were free of clinical heart disease by history and physical examination, using the same inclusion criteria as the Xi’an population.
The study protocols from both centers were drawn up in compliance with the principles of the Helsinki Accord and were reviewed and approved by the local Ethical Committees. Statement of informed consent was obtained from all participants after a full explanation of the procedure.
Genotyping experiments were performed as previously described. Two validated nonsynonymous SNPs (rs17740607 and rs2073440) of HDC gene with certain minor allele frequency (MAF) levels in Chinese Han population according to HapMap data ( http://www.hapmap.org ) were selected and genotyped. See Supplementary Data and Supplementary Table 1 for more details.
Then, we established rs17740607G- or rs17740607A- HDC cDNA constructs and transfected to 293T cells. The HDC transcript levels and protein levels were measured by real-time polymerase chain reaction (PCR) and Western blotting, respectively. Furthermore, The HDC activity was measured by histamine formation method. Histamine concentrations were measured by high-performance liquid chromatography–based o-phthalaldialdehyde (OPA) histamine derivatization method. The detailed protocols are shown in the Supplementary Data .
In separate experiments, the plasma histamine levels of all the participants were also measured by this method. To avoid occasional fluctuation of plasma histamine levels, for each participant, the histamine concentration was calculated as a mean value of 3 fasting plasma samples, respectively, obtained before 3 meals within a day.
Statistical analyses were performed with SPSS 18.0 for Windows (PASW Statistics, SPSS Inc., Chicago, IL). A 2-sided p value <0.05 was considered statistical significance for all tests. Bonferroni corrections were used for multiple comparisons. Each SNP frequency in control subjects was tested for departure from Hardy–Weinberg equilibrium (HWE). The Student t test, analysis of variance, chi-square (Pearson’s chi-square) test or Fisher’s exact test, and multivariate logistic regression analysis or analysis of covariance adjusted for age, gender, body mass index (BMI), and traditional cardiovascular risk factors (hypertension, dyslipidemia, diabetes and smoking habit) under different genetic models were used where necessary. Odds ratios (ORs) with 95% confidential intervals (CIs) were used to assess the associations between genotypes and CHF risk or clinical variables. In addition, a cumulative meta-analysis was conducted for further result validation using Review Manager Software (RevMan, version 5.2.7).
Results
The demographic and clinical characteristics of the study populations are provided in Tables 1 and 2 . In the Xi’an population ( Table 1 ), cases and controls were of similar age and BMI. On the basis of selection criteria, controls had no CHF, no significant coronary artery disease, and a normal average ejection fraction. In contrast, heart failure cases had an advanced systolic heart failure phenotype with significantly decreased ejection fraction. The prevalence of ischemic and nonischemic etiologies was relatively close, whereas the prevalence of all traditional cardiovascular risk factors was significantly greater in patients with CHF in comparison with that observed in the control group, as expected. In the Kunming replication population ( Table 2 ), cases again had severe systolic heart failure. Controls had no CHF based on selection criteria. Furthermore, the prevalence of all traditional risk factors was significantly different between patients and controls.
| CHF ( n = 439) | Control ( n = 467) | P | |
|---|---|---|---|
| Age (years) | 62.6 ± 12.5 | 61.4 ± 8.5 | 0.096 ∗ |
| Men | 291 (66.3%) | 306 (65.5%) | 0.834 † |
| Body mass index (kg/m 2 ) | 24.2 ± 2.8 | 23.8 ± 3.0 | 0.071 ∗ |
| Hypertension ‡ | 263 (59.9%) | 111 (23.8%) | <0.001 † |
| Dyslipidemia § | 150 (34.2%) | 115 (24.6%) | 0.002 † |
| Diabetes mellitus | 135 (30.8%) | 47 (10.1%) | <0.001 † |
| Smokers | 128 (29.2%) | 96 (20.6%) | 0.003 † |
| Left ventricular ejection fraction (%) | 32.9 ± 7.0 | 65.1 ± 6.0 | <0.001 ∗ |
| Etiology of CHF | |||
| Ischemic | 247 (56.3%) | — | |
| Non-ischemic | 192 (43.7%) | — | |
| NYHA class | |||
| II | 156 (35.5%) | — | |
| III | 160 (36.4%) | — | |
| IV | 123 (28.0%) | — |
∗ P values were calculated by student t tests.
† P values were calculated from two-sided chi-square test.
‡ Hypertension was defined as a systolic blood pressure (SBP) of 140 mmHg or a diastolic blood pressure (DBP) of 90 mmHg or those who were receiving antihypertensive therapy at the time of the examination.
§ Dyslipidemia was defined as any of the followings being abnormal: TC, TG, LDL-C, or HDL-C according to Chinese Guidelines on Prevention and Treatment of Dyslipidemia in Adults.
| CHF ( n = 413) | Control ( n = 452) | P | |
|---|---|---|---|
| Age (years) | 61.5 ± 12.0 | 60.8 ± 8.1 | 0.358 ∗ |
| Male | 228 (55.2%) | 231 (51.1%) | 0.246 † |
| Body mass index (kg/m 2 ) | 24.4 ± 2.6 | 24.2 ± 2.6 | 0.178 ∗ |
| Hypertension | 254 (61.5%) | 129 (28.5%) | <0.001 † |
| Dyslipidemia | 164 (39.7%) | 63 (13.9%) | <0.001 † |
| Diabetes mellitus | 137 (33.2%) | 39 (8.6%) | <0.001 † |
| Smokers | 200 (48.4%) | 78 (17.3%) | <0.001 † |
| Left ventricular ejection fraction (%) | 34.5 ± 6.7 | 65.4 ± 6.3 | <0.001 ∗ |
| Etiology of CHF | |||
| Ischemic | 244 (59.1%) | — | |
| Non-ischemic | 169 (40.9%) | — | |
| NYHA class | |||
| II | 96 (23.2%) | — | |
| III | 172 (41.6%) | — | |
| IV | 145 (35.1%) | — |
∗ P values were calculated by student t tests.
The distributions of genotype and allele frequencies of the 2 selected nonsynonymous SNPs in both case–control populations are listed in Table 3 . In the health controls of Xi’an population, neither of the genotype distributions showed any deviance from those expected under HWE (p = 0.238 and 0.358 for rs17740607 and rs2073440, respectively), which was also observed in Kunming population (p = 0.915 and 0.466 for rs17740607 and rs2073440, respectively).
| Genotype | Xi’an CHF | Xi’an Control | P ∗ | Kunming CHF | Kunming Control | P ∗ |
|---|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |||
| rs17740607 | ||||||
| GG | 414 (94.3) | 396 (84.8) | <0.001 | 381 (92.3) | 373 (82.5) | <0.001 |
| GA | 24 (5.5) | 66 (14.1) | 30 (7.3) | 75 (16.6) | ||
| AA | 1 (0.2) | 5 (1.1) | 2 (0.5) | 4 (0.9) | ||
| G/A allele | 852/26 | 858/76 | <0.001 | 792/34 | 821/83 | <0.001 |
| rs2073440 | ||||||
| AA | 374 (85.2) | 401 (85.9) | 0.764 | 348 (84.3) | 378 (83.6) | 0.971 |
| AC | 59 (13.4) | 62 (13.3) | 64 (15.5) | 72 (15.9) | ||
| CC | 6 (1.4) | 4 (0.9) | 1 (0.2) | 2 (0.14) | ||
| A/C allele | 807/71 | 864/70 | 0.661 | 760/66 | 828/76 | 0.793 |
∗ P values were calculated from two-sided chi-square tests or Fisher’s exact tests.
In the following association analysis, we observed a significant difference in both genotype and allele distributions between patients with CHF and controls in Xi’an population for rs17740607 at Bonferroni-corrected p level ( Table 3 ). The A allele was more frequent in control group than in patients with CHF and exhibited protective effect against CHF. These findings were all replicated in Kunming population. To further validate these findings, we combined our rs17740607 genotypic or allelic results of the 2 populations for meta-analysis. The pooled meta-analysis results reveal that the rs17740607 functional polymorphism is significantly associated with the CHF ( Figure 1 ). Meanwhile, for rs2073440, no significant associations with CHF were observed in both populations.
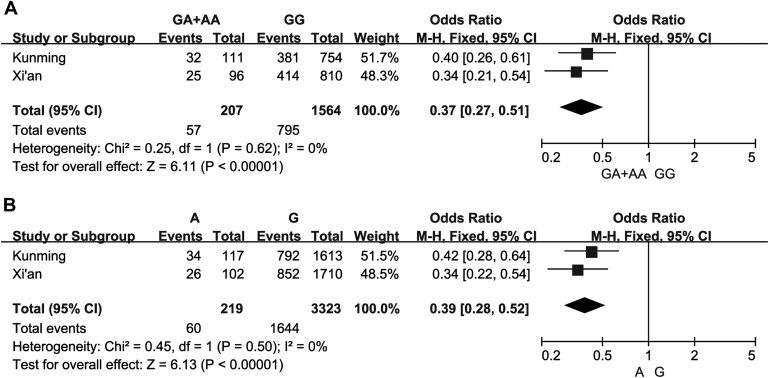
Subsequently, we further performed multivariate analysis for rs17740607 by adjusting for age, gender, BMI, and a series of traditional cardiovascular risk factors under 3 genetic models of inheritance (dominant, recessive, and additive model). After the adjustment, the rs17740607 polymorphism was significantly and independently associated with the predisposition to CHF under a dominant and additive but not recessive model in both populations and consistently exhibited protective effect against CHF according to the values of ORs and 95% CIs ( Table 4 ).
| rs17740607 | Dominant model | Recessive model | Additive model |
|---|---|---|---|
| P ∗ ; OR (95% CI) | P ∗ ; OR (95% CI) | P ∗ ; OR (95% CI) | |
| Xi’an population | <0.001; 0.321 (0.188 −0.547) | 0.159; 0.196 (0.020 – 1.893) | <0.001; 0.346 (0.209 −0.572) |
| Kunming population | 0.002; 0.440 (0.259 −0.747) | 0.632; 0.566 (0.055 – 5.789) | 0.003; 0.471 (0.286 −0.774) |
∗ P values were calculated by logistic regression adjusted for age, gender, body mass index and traditional cardiovascular risk factors.
Then, the genotype distributions of rs17740607 were explored in relation to different CHF clinical subsets ( Table 5 and Supplementary Table 2 ). In the 2 investigated populations, we found no significant difference in genotype distributions for rs17740607 according to functional New York Heart Association (NYHA) class. However, a significantly greater prevalence of the rs17740607G variant was observed in CHF hypertensive patients, diabetic patients, and smokers than in controls in both populations ( Table 5 ). Although the genotype distributions for rs17740607 were observed to be significantly different on the basis of dyslipidemia subsets in Xi’an population, these findings were not replicated in Kunming population. In subsets of patients with CHF without traditional cardiovascular risk factors, the distributions of rs17740607 genotypes still exhibited significant difference with the corresponding health control groups ( Supplementary Table 2 ). We also used all available data to explore whether the strength of association for our findings varied with the underlying cause of CHF (i.e., ischemic and nonischemic). For both populations, the association with rs17740607 was noteworthy in ischemic subtypes, but no association was observed in nonischemic CHF ( Table 5 ).
| rs17740607 (Xi’an population) | P value | rs17740607 (Kunming population) | P value | |||
|---|---|---|---|---|---|---|
| GG ( n = 414) | GA+AA ( n = 25) | GG ( n = 381) | GA+AA ( n = 32) | |||
| NYHA n (%) | ||||||
| II | 144 (34.8) | 12 (48.0) | 0.206 ∗ | 89 (23.4) | 7 (21.9) | 0.332 ∗ |
| III | 155 (37.4) | 5 (20.0) | 162 (42.5) | 10 (31.3) | ||
| IV | 115 (27.8) | 8 (32.0) | 130 (34.1) | 15 (46.9) | ||
| Hypertension | 246 (59.4) | 17 (68.0) | 0.001 † | 247 (64.8) | 7 (21.9) | <0.001 † |
| Dyslipidemia | 143 (34.5) | 7 (28.0) | 0.001 † | 146 (38.3) | 18 (56.3) | 0.060 † |
| Diabetes mellitus | 130 (31.4) | 5 (20.0) | <0.001 † | 125 (32.8) | 12 (37.5) | 0.015 † |
| Smokers | 119 (28.7) | 9 (36.0) | 0.018 † | 179 (47.0) | 21 (65.6) | 0.025 † |
| Ischemic | 240 (58.0) | 7 (28.0) | <0.001 † | 231 (60.6) | 13 (40.6) | <0.001 † |
| Non-ischemic | 174 (42.0) | 18 (72.0) | 0.059 † | 150 (39.4) | 19 (59.4) | 0.064 † |
| LVEF (%) | 33.0 ± 7.0 | 32.4 ± 7.9 | 0.712 ‡ | 34.4 ± 6.4 | 36.2 ± 9.7 | 0.309 ‡ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


